Disruption of β-catenin-mediated negative feedback reinforces cAMP-induced neuronal differentiation in glioma stem cells
- PMID: 35610201
- PMCID: PMC9130142
- DOI: 10.1038/s41419-022-04957-9
Disruption of β-catenin-mediated negative feedback reinforces cAMP-induced neuronal differentiation in glioma stem cells
Abstract
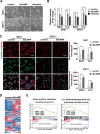
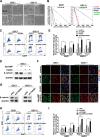
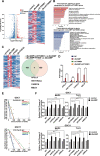
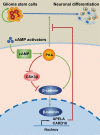
Accumulating evidence supports the existence of glioma stem cells (GSCs) and their critical role in the resistance to conventional treatments for glioblastoma multiforme (GBM). Differentiation therapy represents a promising alternative strategy against GBM by forcing GSCs to exit the cell cycle and reach terminal differentiation. In this study, we demonstrated that cAMP triggered neuronal differentiation and compromised the self-renewal capacity in GSCs. In addition, cAMP induced negative feedback to antagonize the differentiation process by activating β-catenin pathway. Suppression of β-catenin signaling synergized with cAMP activators to eliminate GSCs in vitro and extended the survival of animals in vivo. The cAMP/PKA pathway stabilized β-catenin through direct phosphorylation of the molecule and inhibition of GSK-3β. The activated β-catenin translocated into the nucleus and promoted the transcription of APELA and CARD16, which were found to be responsible for the repression of cAMP-induced differentiation in GSCs. Overall, our findings identified a negative feedback mechanism for cAMP-induced differentiation in GSCs and provided potential targets for the reinforcement of differentiation therapy for GBM.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: A Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 2020;22:1073–113. doi: 10.1093/neuonc/noaa106. - DOI - PMC - PubMed
-
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

