Auditory processing remains sensitive to environmental experience during adolescence in a rodent model
- PMID: 35610222
- PMCID: PMC9130260
- DOI: 10.1038/s41467-022-30455-9
Auditory processing remains sensitive to environmental experience during adolescence in a rodent model
Abstract
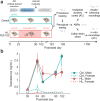
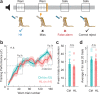
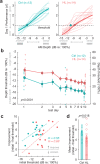
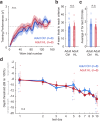
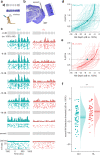
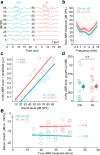
Elevated neural plasticity during development contributes to dramatic improvements in perceptual, motor, and cognitive skills. However, malleable neural circuits are vulnerable to environmental influences that may disrupt behavioral maturation. While these risks are well-established prior to sexual maturity (i.e., critical periods), the degree of neural vulnerability during adolescence remains uncertain. Here, we induce transient hearing loss (HL) spanning adolescence in gerbils, and ask whether behavioral and neural maturation are disrupted. We find that adolescent HL causes a significant perceptual deficit that can be attributed to degraded auditory cortex processing, as assessed with wireless single neuron recordings and within-session population-level analyses. Finally, auditory cortex brain slices from adolescent HL animals reveal synaptic deficits that are distinct from those typically observed after critical period deprivation. Taken together, these results show that diminished adolescent sensory experience can cause long-lasting behavioral deficits that originate, in part, from a dysfunctional cortical circuit.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

