Investigating preparation and characterisation of diphtheria toxoid-loaded on sodium alginate nanoparticles
- PMID: 35610737
- PMCID: PMC9178656
- DOI: 10.1049/nbt2.12088
Investigating preparation and characterisation of diphtheria toxoid-loaded on sodium alginate nanoparticles
Abstract
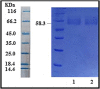
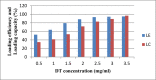
This paper aims to investigate the preparation and characterisation of the alginate nanoparticles (NPs) as antigen delivery system loaded by diphtheria toxoid (DT). For this purpose, both the loading capacity (LC) and Loading efficiency (LE) of the alginate NPs burdened by DT are evaluated. Moreover, the effects of different concentrations of sodium alginate and calcium chloride on the NPs physicochemical characteristics are surveyed in addition to other physical conditions such as homogenization time and rate. To do so, the NPs are characterised using particle size and distribution, zeta potential, scanning electron microscopy, encapsulation efficiency, in vitro release study and FT-IR spectroscopy. Subsequently, the effects of homogenization time and rate on the NPs are assessed. At the meantime, the NPs LC and efficiency in several DT concentrations are estimated. The average size of the NPs was 400.7 and 276.6 nm for unloaded and DT loaded, respectively. According to the obtained results, the zeta potential of the blank and DT loaded NPs are estimated as -23.7 mV and -21.2 mV, respectively. Whereas, the LC and LE were >80% and >90%, in that order. Furthermore, 95% of the releasing DT loaded NPs occurs at 140 h in the sustained mode without any bursting release. It can be concluded that the features of NPs such as morphology and particle size are strongly depended on the calcium chloride, sodium alginate concentrations and physicochemical conditions in the NPs formation process. In addition, appropriate concentrations of the sodium alginate and calcium ions would lead to obtaining the desirable NPs formation associated with the advantageous LE, LC (over 80%) and sustained in vitro release profile. Ultimately, the proposed NPs can be employed in vaccine formulation for the targeted delivery, controlled and slow antigen release associated with the improved antigen stability.
Keywords: antigen delivery system; diphtheria toxoid; loading capacity; loading efficiency; nanoparticles; sodium alginate.
© 2022 The Authors. IET Nanobiotechnology published by John Wiley & Sons Ltd on behalf of The Institution of Engineering and Technology.
Conflict of interest statement
The authors declare that they have no conflict of interest(s).
Figures




References
-
- Mehrabi, M. , et al.: Development and optimisation of hepatitis B recombinant antigen loaded chitosan nanoparticles as an adjuvant using the response surface methodology. Micro & Nano Lett. 15(11), 736–741 (2020). 10.1049/mnl.2019.0355 - DOI
-
- Akagi, T. , Baba, M. , Akashi, M. : Biodegradable nanoparticles as vaccine adjuvants and delivery systems: regulation of immune responses by nanoparticle‐based vaccine. In: Kunugi, S. , Yamaoka, T. (eds.) Polymers in Nanomedicine. Advances in Polymer Science, vol. 247, pp. 31–64. Springer, Berlin, Heidelberg (2011). 10.1007/12_2011_150 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

