Transcriptomics-Based Investigation of Molecular Mechanisms Underlying Apoptosis Induced by ZnO Nanoparticles in Human Diffuse Large B-Cell Lymphoma
- PMID: 35611214
- PMCID: PMC9124502
- DOI: 10.2147/IJN.S355408
Transcriptomics-Based Investigation of Molecular Mechanisms Underlying Apoptosis Induced by ZnO Nanoparticles in Human Diffuse Large B-Cell Lymphoma
Abstract
Introduction: Zinc oxide nanoparticles (ZnO NPs) show anti-cancer activity. Diffuse Large B-cell Lymphoma (DLBCL) is a type of B-cell malignancies with unsatisfying treatment outcomes. This study was set to assess the potential of ZnO NPs to selectively induce apoptosis in human DLBCL cells (OCI-LY3), and to describe possible molecular mechanisms of action.
Methods: The impact of ZnO NPs on DLBCL cells and normal peripheral blood mononuclear cells (PBMCs) was studied using cytotoxicity assay and flow-cytometry. Transcriptomics analysis was conducted to identify ZnO NPs-dependent changes in the transcriptomic profiles of DLBCL cells.
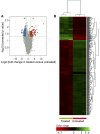
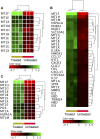
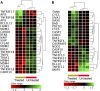
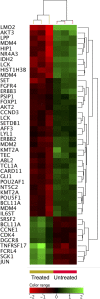
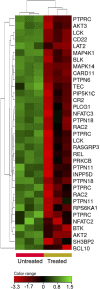
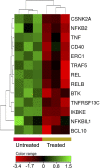
Results: ZnO NPs selectively induced apoptosis in DLBCL cells, and caused changes in their transcriptomes. Deferential gene expression (DGE) with fold change (FC) ≥3 and p ≤ 0.008 with corrected p ≤ 0.05 was identified for 528 genes; 125 genes were over-expressed and 403 genes were under-expressed in ZnO NPs-treated DLBCL cells. The over-expressed genes involved in biological processes and pathways like stress response to metal ion, cellular response to zinc ion, metallothioneins bind metals, oxidative stress, and negative regulation of growth. In contrast, the under-expressed genes were implicated in DNA packaging complex, signaling by NOTCH, negative regulation of gene expression by epigenetic, signaling by WNT, M phase of cell cycle, and telomere maintenance. Setting the FC to ≥1.5 with p ≤ 0.05 and corrected p ≤ 0.1 showed ZnO NPs to induce over-expression of anti-oxidant genes and under-expression of oncogenes; target B-cell receptor (BCR) signaling pathway and NF-κB pathway; and promote apoptosis by intrinsic and extrinsic pathways.
Discussion: Overall, ZnO NPs selectively induced apoptosis in DLBCL cells, and possible molecular mechanisms of action were described.
Keywords: DLBCL; ZnO NPs; apoptosis; mechanism of action; transcriptomics.
© 2022 Alsagaby.
Conflict of interest statement
The author declares that there are no conflicts of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

