Role of Aducanumab in the Treatment of Alzheimer's Disease: Challenges and Opportunities
- PMID: 35611326
- PMCID: PMC9124475
- DOI: 10.2147/CIA.S325026
Role of Aducanumab in the Treatment of Alzheimer's Disease: Challenges and Opportunities
Abstract
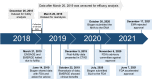
Aducanumab is a monoclonal antibody selective for amyloid β (Aβ) aggregates. In June 2021, aducanumab became the first drug underlying the pathophysiology of Alzheimer's disease (AD) approved by the US Food and Drug Administration (FDA), under the accelerated approval pathway. The decision was based on the ability of aducanumab to remove Aβ plaques, without any evidence that the Aβ clearance is correlated with less cognitive or functional decline. This decision has generated a considerable debate in the scientific community, especially because the results from the two Phase 3 trials, EMERGE and ENGAGE, were divergent and, even after the post hoc analysis, the data were insufficient to prove aducanumab efficacy. Moreover, some researchers think that this approval will be an obstacle to the progress and also demonstrated concerns about aducanumab cost and its safety profile. The European Medicines Agency's rejection of aducanumab in December 2021 just brought more controversy over FDA's decision. Now, Biogen is designing the FDA's required confirmatory study, named ENVISION, which should be complete in 2026. Despite the controversy, the aducanumab showed to affect downstream tau pathology, which could open doors for a combination therapy approach for AD (anti-tau and anti-amyloid drug). This review summarizes the clinical development of aducanumab until regulatory agencies' decisions, the available trials data and the controversy over aducanumab approval for AD.
Keywords: ARIA; European Medicines Agency; Food and Drug Administration; anti-Aβ monoclonal antibody; clinical trials; tau protein.
© 2022 Vaz et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- World Health Organization. Global action plan on the public health response to dementia 2017–2025. Geneva:World Heal Organ; 2017: 27. Available from: http://www.who.int/mental_health/neurology/dementia/action_plan_2017_202.... Accessed May 14, 2022.
-
- U.S. Food and Drug Administration (FDA). FDA grants accelerated approval for Alzheimer’s drug. FDA News Release; 2021. Available from: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerat.... Accessed November 14, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical