A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease
- PMID: 35611346
- PMCID: PMC9047227
- DOI: 10.1093/ofid/ofac138
A Systematic Review of Coronavirus Disease 2019 Vaccine Efficacy and Effectiveness Against Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Disease
Abstract
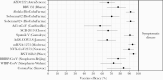
Billions of doses of coronavirus disease 2019 (COVID-19) vaccines have been administered globally, dramatically reducing severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) incidence and severity in some settings. Many studies suggest vaccines provide a high degree of protection against infection and disease, but precise estimates vary and studies differ in design, outcomes measured, dosing regime, location, and circulating virus strains. In this study, we conduct a systematic review of COVID-19 vaccines through February 2022. We included efficacy data from Phase 3 clinical trials for 15 vaccines undergoing World Health Organization Emergency Use Listing evaluation and real-world effectiveness for 8 vaccines with observational studies meeting inclusion criteria. Vaccine metrics collected include protection against asymptomatic infection, any infection, symptomatic COVID-19, and severe outcomes including hospitalization and death, for partial or complete vaccination, and against variants of concern Alpha, Beta, Gamma, Delta, and Omicron. We additionally review the epidemiological principles behind the design and interpretation of vaccine efficacy and effectiveness studies, including important sources of heterogeneity.
Keywords: COVID-19; SARS-CoV-2; systematic review; vaccine effectiveness; vaccine efficacy.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures






References
-
- Mathieu E, Ritchie H, Ortiz-Ospina E, et al. . A global database of COVID-19 vaccinations. Nat Hum Behav 2021; 5:947–53. - PubMed
-
- Basta N, Moodie E; McGill University COVID19 Vaccine Tracker Team. COVID-19 Vaccine Development and Approvals Tracker. COVID19 Vaccine Tracker. Available at: https://covid19.trackvaccines.org/. Accessed 26 August 2021.
-
- Stowe J, Andrews N, Gower C, et al. . Effectiveness of COVID-19 vaccines against hospital admission with the Delta (B.1.617.2) variant. Available at: https://khub.net/documents/135939561/479607266/Effectiveness+of+COVID-19.... Accessed 22 June 2021.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

