Delayed hospitalisation for heart failure after transcatheter repair or medical treatment for secondary mitral regurgitation: a landmark analysis of the MITRA-FR trial
- PMID: 35611516
- PMCID: PMC10241268
- DOI: 10.4244/EIJ-D-21-00846
Delayed hospitalisation for heart failure after transcatheter repair or medical treatment for secondary mitral regurgitation: a landmark analysis of the MITRA-FR trial
Abstract
Background: In the MITRA-FR trial, transcatheter mitral valve repair (TMVR) was not associated with a 2-year clinical benefit in patients with secondary mitral regurgitation (SMR).
Aims: This landmark analysis aimed at investigating a potential reduction of the hospitalisation rate for heart failure (HF) between 12 and 24 months after inclusion in the MITRA-FR trial in patients randomised to the intervention group (TMVR with the MitraClip device), as compared with patients randomised to the control group (guideline-directed medical therapy [GDMT]).
Methods: The MITRA-FR trial randomised 307 patients with SMR for TMVR on top of GDMT (TMVR group; n=152) or for GDMT alone (control group; n=155). We conducted a 12-month landmark analysis in surviving patients who were not hospitalised for HF within the first 12 months of follow-up. The primary endpoint was the 1-year cumulative number of HF hospitalisations.
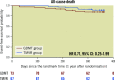
Results: A total of 140 patients (TMVR group: 67; GDMT group: 73) were selected for this landmark analysis with similar characteristics at inclusion in the trial. The primary endpoint was 28 events per 100 patient-years in the TMVR group, as compared with 60 events per 100 patient-years in the GDMT group (hazard ratio [HR] 0.46, 95% confidence interval [CI]: 0.20-1.02; p=0.057).
Conclusions: In this landmark analysis of the MITRA-FR trial, the cumulative rate of HF hospitalisation between 12 and 24 months among patients treated with TMVR on top of GDMT was approximately half as many as those of patients treated with GDMT alone, a difference which did not reach statistical significance in the setting of a low number of events.
Conflict of interest statement
G. Leurent reports proctoring activity and received lecture and consultant fees from Abbott. V. Auffret received lecture fees from Medtronic and BMS/Pfizer. E. Donal received research grants from General Electric and Abbott. G. Bonnet received proctoring fees from Abbott. P.Y. Leroux reports proctoring fees from Abbott. P. Guerin reports proctoring fees from Abbott; received research grants from Abbott and Edwards Lifesciences. T. Lefèvre received proctoring fees from Abbott. D. Messika-Zeitoun received research grants from Edwards Lifesciences.B. Iung received lecture fees from Edwards Lifesciences. J.N. Trochu received institutional grants from Boston Scientific and Novartis and personal/consulting fees from Abbott, Bayer, Novartis, Boston Scientific and Vifor Pharma. J.F. Obadia received consultant fees from Abbott, Carmat, Delacroix-Chevalier, Medtronic and Landanger. The other authors have no conflicts of interest to declare.
Figures




References
-
- Obadia JF, Messika-Zeitoun D, Leurent G, Iung B, Bonnet G, Piriou N, Lefèvre T, Piot C, Rouleau F, Carrié D, Nejjari M, Ohlmann P, Leclercq F, Saint Etienne, Teiger E, Leroux L, Karam N, Michel N, Gilard M, Donal E, Trochu JN, Cormier B, Armoiry X, Boutitie F, Maucort-Boulch D, Barnel C, Samson G, Guerin P, Vahanian A, Mewton N MITRA-FR Investigators. Percutaneous Repair or Medical Treatment for Secondary Mitral Regurgitation. N Engl J Med. 2018;379:2297–306. - PubMed
-
- Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ COAPT Investigators. Transcatheter Mitral-Valve Repair in Patients with Heart Failure. N Engl J Med. 2018;379:2307–18. - PubMed
-
- Iung B, Armoiry X, Vahanian A, Boutitie F, Mewton N, Trochu JN, Lefèvre T, Messika-Zeitoun D, Guerin P, Cormier B, Brochet E, Thibault H, Himbert D, Thivolet S, Leurent G, Bonnet G, Donal E, Piriou N, Piot C, Habib G, Rouleau F, Carrie D, Nejjari M, Ohlmann P, Saint Etienne, Leroux L, Gilard M, Samson G, Rioufol G, Maucort-Boulch D, Obadia JF, MITRA-FR Investigators. Percutaneous repair or medical treatment for secondary mitral regurgitation: outcomes at 2 years. Eur J Heart Fail. 2019;21:1619–27. - PubMed
-
- Senni M, Adamo M, Metra M, Alfieri O, Vahanian A. Treatment of functional mitral regurgitation in chronic heart failure: can we get a ‘proof of concept’ from the MITRA-FR and COAPT trials? Eur J Heart Fail. 2019;21:852–61. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

