Measuring Cytoskeletal Mechanical Fluctuations and Rheology with Active Micropost Arrays
- PMID: 35612274
- PMCID: PMC9321978
- DOI: 10.1002/cpz1.433
Measuring Cytoskeletal Mechanical Fluctuations and Rheology with Active Micropost Arrays
Abstract
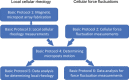
The dynamics of the cellular actomyosin cytoskeleton are crucial to many aspects of cellular function. Here, we describe techniques that employ active micropost array detectors (AMPADs) to measure cytoskeletal rheology and mechanical force fluctuations. The AMPADS are arrays of flexible poly(dimethylsiloxane) (PDMS) microposts with magnetic nanowires embedded in a subset of microposts to enable actuation of those posts via an externally applied magnetic field. Techniques are described to track the magnetic microposts' motion with nanometer precision at up to 100 video frames per second to measure the local cellular rheology at well-defined positions. Application of these high-precision tracking techniques to the full array of microposts in contact with a cell also enables mapping of the cytoskeletal mechanical fluctuation dynamics with high spatial and temporal resolution. This article describes (1) the fabrication of magnetic micropost arrays, (2) measurement protocols for both local rheology and cytoskeletal force fluctuation mapping, and (3) special-purpose software routines to reduce and analyze these data. © 2022 The Authors. Current Protocols published by Wiley Periodicals LLC. Basic Protocol 1: Fabrication of magnetic micropost arrays Basic Protocol 2: Data acquisition for cellular force fluctuations on non-magnetic micropost arrays Basic Protocol 3: Data acquisition for local cellular rheology measurements with magnetic microposts Basic Protocol 4: Data reduction: determining microposts' motion Basic Protocol 5: Data analysis: determining local rheology from magnetic microposts Basic Protocol 6: Data analysis for force fluctuation measurements Support Protocol 1: Fabrication of magnetic Ni nanowires by electrodeposition Support Protocol 2: Configuring Streampix for magnetic rheology measurements.
Keywords: cytoskeleton; magnetic actuation; microposts; microrheology.
© 2022 The Authors. Current Protocols published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





















Similar articles
-
Easy and Accurate Mechano-profiling on Micropost Arrays.J Vis Exp. 2015 Nov 17;(105):53350. doi: 10.3791/53350. J Vis Exp. 2015. PMID: 26650118 Free PMC article.
-
Magnetic microposts for mechanical stimulation of biological cells: fabrication, characterization, and analysis.Rev Sci Instrum. 2008 Apr;79(4):044302. doi: 10.1063/1.2906228. Rev Sci Instrum. 2008. PMID: 18447536 Free PMC article.
-
PDMS Elastic Micropost Arrays for Studying Vascular Smooth Muscle Cells.Sens Actuators B Chem. 2013 Nov 1;188:1055-1063. doi: 10.1016/j.snb.2013.08.018. Sens Actuators B Chem. 2013. PMID: 26451074 Free PMC article.
-
For whom the cells pull: Hydrogel and micropost devices for measuring traction forces.Methods. 2016 Feb 1;94:51-64. doi: 10.1016/j.ymeth.2015.08.005. Epub 2015 Aug 8. Methods. 2016. PMID: 26265073 Free PMC article. Review.
-
Application of Microrheology in Food Science.Annu Rev Food Sci Technol. 2017 Feb 28;8:493-521. doi: 10.1146/annurev-food-030216-025859. Epub 2017 Jan 12. Annu Rev Food Sci Technol. 2017. PMID: 28125345 Review.
Cited by
-
All-Covalent Nuclease-Resistant and Hydrogel-Tethered DNA Hairpin Probes Map pN Cell Traction Forces.ACS Appl Mater Interfaces. 2023 Jul 19;15(28):33362-33372. doi: 10.1021/acsami.3c04826. Epub 2023 Jul 6. ACS Appl Mater Interfaces. 2023. PMID: 37409737 Free PMC article.
References
Literature Cited
-
- Crocker, J. C. , & Grier, D. G. (1996). Methods of digital video microscopy for colloidal studies. Journal of Colloid and Interface Science, 179(1), 298–310. doi: 10.1006/jcis.1996.0217 - DOI
-
- Dixon, P. K. , & Wu, L. (1989). Broad‐band digital lock‐in amplifier techniques. Review of Scientific Instruments, 60(10), 3329–3336. doi: 10.1063/1.1140523 - DOI
Internet Resources
-
- The Streampix module files and Igor Pro procedure files needed to run experiments and analyze data are available on Github at https://github.com/yushi1898/MPAD‐Analysis‐Code.
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

