Cyclic di-GMP Regulates the Type III Secretion System and Virulence in Bordetella bronchiseptica
- PMID: 35612302
- PMCID: PMC9202433
- DOI: 10.1128/iai.00107-22
Cyclic di-GMP Regulates the Type III Secretion System and Virulence in Bordetella bronchiseptica
Abstract
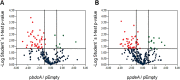
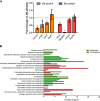
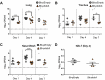
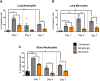
The second messenger cyclic di-GMP (c-di-GMP) is a ubiquitous molecule in bacteria that regulates diverse phenotypes. Among them, motility and biofilm formation are the most studied. Furthermore, c-di-GMP has been suggested to regulate virulence factors, making it important for pathogenesis. Previously, we reported that c-di-GMP regulates biofilm formation and swimming motility in Bordetella bronchiseptica. Here, we present a multi-omics approach for the study of B. bronchiseptica strains expressing different cytoplasmic c-di-GMP levels, including transcriptome sequencing (RNA-seq) and shotgun proteomics with label-free quantification. We detected 64 proteins significantly up- or downregulated in either low or high c-di-GMP levels and 358 genes differentially expressed between strains with high c-di-GMP levels and the wild-type strain. Among them, we found genes for stress-related proteins, genes for nitrogen metabolism enzymes, phage-related genes, and virulence factor genes. Interestingly, we observed that a virulence factor like the type III secretion system (TTSS) was regulated by c-di-GMP. B. bronchiseptica with high c-di-GMP levels showed significantly lower levels of TTSS components like Bsp22, BopN, and Bcr4. These findings were confirmed by independent methods, such as quantitative reverse transcription-PCR (q-RT-PCR) and Western blotting. Higher intracellular levels of c-di-GMP correlated with an impaired capacity to induce cytotoxicity in a eukaryotic cell in vitro and with attenuated virulence in a murine model. This work presents data that support the role that the second messenger c-di-GMP plays in the pathogenesis of Bordetella.
Keywords: Bordetella; RNA-seq; c-di-GMP; immune response; label-free proteomics; type III secretion system.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
BvgR is important for virulence-related phenotypes in Bordetella bronchiseptica.Microbiol Spectr. 2024 Nov 5;12(11):e0079424. doi: 10.1128/spectrum.00794-24. Epub 2024 Oct 4. Microbiol Spectr. 2024. PMID: 39365045 Free PMC article.
-
Interplay of virulence factors and signaling molecules: albumin and calcium-mediated biofilm regulation in Bordetella bronchiseptica.J Bacteriol. 2025 Apr 17;207(4):e0044524. doi: 10.1128/jb.00445-24. Epub 2025 Mar 26. J Bacteriol. 2025. PMID: 40135913 Free PMC article.
-
Bordetella bronchiseptica Diguanylate Cyclase BdcA Regulates Motility and Is Important for the Establishment of Respiratory Infection in Mice.J Bacteriol. 2019 Aug 8;201(17):e00011-19. doi: 10.1128/JB.00011-19. Print 2019 Sep 1. J Bacteriol. 2019. PMID: 31209073 Free PMC article.
-
Cyclic diguanylate differentially regulates the expression of virulence factors and pathogenesis-related phenotypes in Clostridioides difficile.Microbiol Res. 2024 Sep;286:127811. doi: 10.1016/j.micres.2024.127811. Epub 2024 Jun 20. Microbiol Res. 2024. PMID: 38909416 Review.
-
Targeting c-di-GMP Signaling, Biofilm Formation, and Bacterial Motility with Small Molecules.Methods Mol Biol. 2017;1657:419-430. doi: 10.1007/978-1-4939-7240-1_31. Methods Mol Biol. 2017. PMID: 28889311 Review.
Cited by
-
Cyclic di-GMP Signaling Links Biofilm Formation and Mn(II) Oxidation in Pseudomonas resinovorans.mBio. 2022 Dec 20;13(6):e0273422. doi: 10.1128/mbio.02734-22. Epub 2022 Nov 14. mBio. 2022. PMID: 36374078 Free PMC article.
-
Bordetella bronchiseptica diguanylate cyclase BdcB inhibits the type three secretion system and impacts the immune response.Sci Rep. 2023 May 2;13(1):7157. doi: 10.1038/s41598-023-34106-x. Sci Rep. 2023. PMID: 37130958 Free PMC article.
-
Conquering the host: Bordetella spp. and Pseudomonas aeruginosa molecular regulators in lung infection.Front Microbiol. 2022 Sep 26;13:983149. doi: 10.3389/fmicb.2022.983149. eCollection 2022. Front Microbiol. 2022. PMID: 36225372 Free PMC article. Review.
-
Limited response of primary nasal epithelial cells to Bordetella pertussis infection.Microbiol Spectr. 2025 Sep 2;13(9):e0126725. doi: 10.1128/spectrum.01267-25. Epub 2025 Aug 4. Microbiol Spectr. 2025. PMID: 40757824 Free PMC article.
-
Cyclic Diguanylate in the Wild: Roles During Plant and Animal Colonization.Annu Rev Microbiol. 2024 Nov;78(1):533-551. doi: 10.1146/annurev-micro-041522-101729. Epub 2024 Nov 7. Annu Rev Microbiol. 2024. PMID: 39270684 Free PMC article. Review.
References
-
- Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MTG, Churcher CM, Bentley SD, Mungall KL, Cerdeño-Tárraga AM, Temple L, James K, Harris B, Quail MA, Achtman M, Atkin R, Baker S, Basham D, Bason N, Cherevach I, Chillingworth T, Collins M, Cronin A, Davis P, Doggett J, Feltwell T, Goble A, Hamlin N, Hauser H, Holroyd S, Jagels K, Leather S, Moule S, Norberczak H, O'Neil S, Ormond D, Price C, Rabbinowitsch E, Rutter S, Sanders M, Saunders D, Seeger K, Sharp S, Simmonds M, Skelton J, Squares R, Squares S, Stevens K, Unwin L, et al. . 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet 35:32–40. 10.1038/ng1227. - DOI - PubMed
-
- van Gent M, Heuvelman CJ, van der Heide HG, Hallander HO, Advani A, Guiso N, Wirsing von Kőnig CH, Vestrheim DF, Dalby T, Fry NK, Pierard D, Detemmerman L, Zavadilova J, Fabianova K, Logan C, Habington A, Byrne M, Lutyńska A, Mosiej E, Pelaz C, Gröndahl-Yli-Hannuksela K, Barkoff AM, Mertsola J, Economopoulou A, He Q, Mooi FR. 2015. Analysis of Bordetella pertussis clinical isolates circulating in European countries during the period 1998–2012. Eur J Clin Microbiol Infect Dis 34:821–830. 10.1007/s10096-014-2297-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

