Development and Implementation of Dried Blood Spot-Based COVID-19 Serological Assays for Epidemiologic Studies
- PMID: 35612315
- PMCID: PMC9241704
- DOI: 10.1128/spectrum.02471-21
Development and Implementation of Dried Blood Spot-Based COVID-19 Serological Assays for Epidemiologic Studies
Abstract
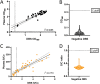
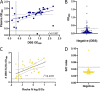
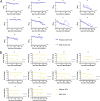
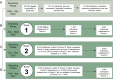
Serological surveillance studies of infectious diseases provide population-level estimates of infection and antibody prevalence, generating crucial insight into population-level immunity, risk factors leading to infection, and effectiveness of public health measures. These studies traditionally rely on detection of pathogen-specific antibodies in samples derived from venipuncture, an expensive and logistically challenging aspect of serological surveillance. During the COVID-19 pandemic, guidelines implemented to prevent the spread of SARS-CoV-2 infection made collection of venous blood logistically difficult at a time when SARS-CoV-2 serosurveillance was urgently needed. Dried blood spots (DBS) have generated interest as an alternative to venous blood for SARS-CoV-2 serological applications due to their stability, low cost, and ease of collection; DBS samples can be self-generated via fingerprick by community members and mailed at ambient temperatures. Here, we detail the development of four DBS-based SARS-CoV-2 serological methods and demonstrate their implementation in a large serological survey of community members from 12 cities in the East Bay region of the San Francisco metropolitan area using at-home DBS collection. We find that DBS perform similarly to plasma/serum in enzyme-linked immunosorbent assays and commercial SARS-CoV-2 serological assays. In addition, we show that DBS samples can reliably detect antibody responses months postinfection and track antibody kinetics after vaccination. Implementation of DBS enabled collection of valuable serological data from our study population to investigate changes in seroprevalence over an 8-month period. Our work makes a strong argument for the implementation of DBS in serological studies, not just for SARS-CoV-2, but any situation where phlebotomy is inaccessible. IMPORTANCE Estimation of community-level antibody responses to SARS-CoV-2 from infection or vaccination is critical to inform public health responses. Traditional studies of antibodies rely on collection of blood via venipuncture, an invasive procedure not amenable to pandemic-related social-distancing measures. Dried blood spots (DBS) are an alternative to venipuncture, since they can be self-collected by study participants at home and do not require refrigeration for shipment or storage. However, DBS-based assays to measure antibody levels to SARS-CoV-2 have not been widely utilized. Here, we show that DBS are comparable to blood as a sampling method for antibody responses to SARS-CoV-2 infection and vaccination over time measured using four distinct serological assays. The DBS format enabled antibody surveillance in a longitudinal cohort where study participants self-collected samples, ensuring the participants' safety during an ongoing pandemic. Our work demonstrates that DBS are an excellent sampling method for measuring antibody responses whenever venipuncture is impractical.
Keywords: COVID-19; SARS-CoV-2; antibodies; dried blood spot; serology; seroprevalence.
Conflict of interest statement
The authors declare a conflict of interest. Brett M. Hirsch and Paul Contestable declare a conflict of interest; both are employees of Ortho Clinical Diagnostics. Vitalant (Michael P. Busch and Mars Stone) receives free and discounted reagents and testing for research studies from Ortho and Roche, but no direct funding as grants or personal compensation from either company.
Figures


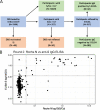
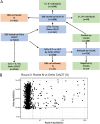
References
-
- World Health Organization. 2020. WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int/.
-
- Stefic K, Guinard J, Peytavin G, Saboni L, Sommen C, Sauvage C, Lot F, Laperche S, Velter A, Barin F. 2019. Screening for human immunodeficiency virus infection by use of a fourth-generation antigen/antibody assay and dried blood spots: in-depth analysis of sensitivity and performance assessment in a cross-sectional study. J Clin Microbiol 58:e01645-19. doi: 10.1128/JCM.01645-19. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

