Delivery of RNA Therapeutics: The Great Endosomal Escape!
- PMID: 35612432
- PMCID: PMC9595607
- DOI: 10.1089/nat.2022.0004
Delivery of RNA Therapeutics: The Great Endosomal Escape!
Abstract
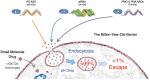
RNA therapeutics, including siRNAs, antisense oligonucleotides, and other oligonucleotides, have great potential to selectively treat a multitude of human diseases, from cancer to COVID to Parkinson's disease. RNA therapeutic activity is mechanistically driven by Watson-Crick base pairing to the target gene RNA without the requirement of prior knowledge of the protein structure, function, or cellular location. However, before widespread use of RNA therapeutics becomes a reality, we must overcome a billion years of evolutionary defenses designed to keep invading RNAs from entering cells. Unlike small-molecule therapeutics that are designed to passively diffuse across the cell membrane, macromolecular RNA therapeutics are too large, too charged, and/or too hydrophilic to passively diffuse across the cellular membrane and are instead taken up into cells by endocytosis. However, similar to the cell membrane, endosomes comprise a lipid bilayer that entraps 99% or more of RNA therapeutics, even in semipermissive tissues such as the liver, central nervous system, and muscle. Consequently, before RNA therapeutics can achieve their ultimate clinical potential to treat widespread human disease, the rate-limiting delivery problem of endosomal escape must be solved in a clinically acceptable manner.
Keywords: ASO; RNA therapeutics; delivery; endosomal escape; siRNA.
Conflict of interest statement
S.F.D. is a member of the Scientific Advisory Boards of Ceptur Therapeutics, Deep Genomics, Generation Bio, Korro Bio, and NeuBase Therapeutics and cofounder of Clear Skies Bio.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

