Apixaban or Warfarin and Aspirin or Placebo After Acute Coronary Syndrome or Percutaneous Coronary Intervention in Patients With Atrial Fibrillation and Prior Stroke: A Post Hoc Analysis From the AUGUSTUS Trial
- PMID: 35612866
- PMCID: PMC9134037
- DOI: 10.1001/jamacardio.2022.1166
Apixaban or Warfarin and Aspirin or Placebo After Acute Coronary Syndrome or Percutaneous Coronary Intervention in Patients With Atrial Fibrillation and Prior Stroke: A Post Hoc Analysis From the AUGUSTUS Trial
Abstract
Importance: Data are limited regarding the risk of cerebrovascular ischemic events and major bleeding in patients with atrial fibrillation (AF) and recent acute coronary syndrome (ACS) and/or percutaneous coronary intervention (PCI).
Objective: Determine the efficacy and safety of apixaban or vitamin K antagonists (VKA) and aspirin or placebo according to prior stroke, transient ischemic attack (TIA), or thromboembolism (TE).
Design, setting, and participants: In this prospective, multicenter, 2-by-2 factorial, randomized clinical trial, post hoc parallel analyses were performed to compare randomized treatment regimens according to presence or absence of prior stroke/TIA/TE using Cox proportional hazards models. Patients with AF, recent ACS or PCI, and planned use of P2Y12 inhibitors for 6 months or longer were included; 33 patients with missing data about prior stroke/TIA/TE were excluded.
Interventions: Apixaban (5 mg or 2.5 mg twice daily) or VKA and aspirin or placebo.
Main outcomes and measures: Major or clinically relevant nonmajor (CRNM) bleeding.
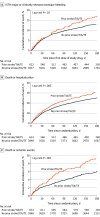
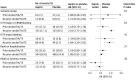
Results: Of 4581 patients included, 633 (13.8%) had prior stroke/TIA/TE. Patients with vs without prior stroke/TIA/TE were older; had higher CHA2DS2-VASC and HAS-BLED scores; and more frequently had prior bleeding, heart failure, diabetes, and prior oral anticoagulant use. Apixaban was associated with lower rates of major or CRNM bleeding and death or hospitalization than VKA in patients with (hazard ratio [HR], 0.69; 95% CI, 0.46-1.03) and without (HR, 0.68; 95% CI, 0.57-0.82) prior stroke/TIA/TE. Patients without prior stroke/TIA/TE receiving aspirin vs placebo had higher rates of bleeding; this difference appeared less substantial among patients with prior stroke/TIA/TE (P = .01 for interaction). Aspirin was associated with numerically lower rates of death or ischemic events than placebo in patients with (HR, 0.71; 95% CI, 0.42-1.20) and without (HR, 0.93; 95% CI, 0.72-1.21) prior stroke/TIA/TE (not statistically significant).
Conclusions and relevance: The safety and efficacy of apixaban compared with VKA was consistent with the AUGUSTUS findings, irrespective of prior stroke/TIA/TE. Aspirin increased major or CRNM bleeding, particularly in patients without prior stroke/TIA/TE. Although aspirin may have some benefit in patients with prior stroke, our findings support the use of apixaban and a P2Y12 inhibitor without aspirin for the majority of patients with AF and ACS and/or PCI, regardless of prior stroke/TIA/TE status.
Trial registration: ClinicalTrials.gov Identifier: NCT02415400.
Conflict of interest statement
Figures

References
-
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272. doi: 10.1378/chest.09-1584 - DOI - PubMed
-
- Easton JD, Lopes RD, Bahit MC, et al. ; ARISTOTLE Committees and Investigators . Apixaban compared with warfarin in patients with atrial fibrillation and previous stroke or transient ischaemic attack: a subgroup analysis of the ARISTOTLE trial. Lancet Neurol. 2012;11(6):503-511. doi: 10.1016/S1474-4422(12)70092-3 - DOI - PubMed
-
- Myint PK, Kwok CS, Roffe C, et al. ; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research . Determinants and outcomes of stroke following percutaneous coronary intervention by indication. Stroke. 2016;47(6):1500-1507. doi: 10.1161/STROKEAHA.116.012700 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

