Durability of the Treatment Effects of an 8-Week Self-administered Home-Based Virtual Reality Program for Chronic Low Back Pain: 6-Month Follow-up Study of a Randomized Clinical Trial
- PMID: 35612905
- PMCID: PMC9177046
- DOI: 10.2196/37480
Durability of the Treatment Effects of an 8-Week Self-administered Home-Based Virtual Reality Program for Chronic Low Back Pain: 6-Month Follow-up Study of a Randomized Clinical Trial
Erratum in
-
Correction: Durability of the Treatment Effects of an 8-Week Self-administered Home-Based Virtual Reality Program for Chronic Low Back Pain: 6-Month Follow-up Study of a Randomized Clinical Trial.J Med Internet Res. 2022 Jun 8;24(6):e40038. doi: 10.2196/40038. J Med Internet Res. 2022. PMID: 35675658 Free PMC article.
Abstract
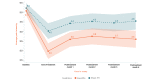
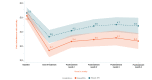
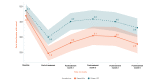
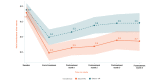
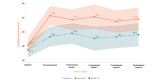
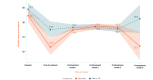
Background: We previously reported the efficacy of an 8-week home-based therapeutic immersive virtual reality (VR) program in a double-blind randomized placebo-controlled study. Community-based adults with self-reported chronic low back pain were randomized 1:1 to receive either (1) a 56-day immersive therapeutic pain relief skills VR program (EaseVRx) or (2) a 56-day sham VR program. Immediate posttreatment results revealed the superiority of therapeutic VR over sham VR for reducing pain intensity; pain-related interference with activity, mood, and stress (but not sleep); physical function; and sleep disturbance. At 3 months posttreatment, therapeutic VR maintained superiority for reducing pain intensity and pain-related interference with activity, stress, and sleep (new finding).
Objective: This study assessed between-group and within-group treatment effects 6 months posttreatment to determine the extended efficacy, magnitude of efficacy, and clinical importance of home-based therapeutic VR.
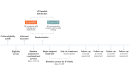
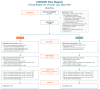
Methods: E-surveys were deployed at pretreatment, end-of-treatment, and posttreatment months 1, 2, 3, and 6. Self-reported data for 188 participants were analyzed in a mixed-model framework using a marginal model to allow for correlated responses across the repeated measures. Primary outcomes were pain intensity and pain-related interference with activity, mood, stress, and sleep at 6 months posttreatment. Secondary outcomes were Patient-Reported Outcome Measurement Information System (PROMIS) sleep disturbance and physical function.
Results: Therapeutic VR maintained significant and clinically meaningful effects 6 months posttreatment and remained superior to sham VR for reducing pain intensity and pain-related interference with activity, stress, and sleep (ds=0.44-0.54; P<.003). Between-group comparisons for physical function and sleep disturbance showed superiority of EaseVRx over sham VR (ds=0.34; P=.02 and ds=0.46; P<.001, respectively). Participants were encouraged to contact study staff with any problems experienced during treatment; however, no participants contacted study staff to report adverse events of any type, including nausea and motion sickness.
Conclusions: Our 8-week home-based VR pain management program caused important reductions in pain intensity and interference up to 6 months after treatment. Additional studies are needed in diverse samples.
Trial registration: ClinicalTrials.gov NCT04415177; https://clinicaltrials.gov/ct2/show/NCT04415177.
International registered report identifier (irrid): RR2-10.2196/25291.
Keywords: behavioral health; chronic low back pain; treatment; virtual reality.
©Laura Garcia, Brandon Birckhead, Parthasarathy Krishnamurthy, Ian Mackey, Josh Sackman, Vafi Salmasi, Robert Louis, Carina Castro, Roselani Maddox, Todd Maddox, Beth D Darnall. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 25.05.2022.
Conflict of interest statement
Conflicts of Interest: TM and RM are employees of AppliedVR, Inc. JS is the president of AppliedVR, Inc. BB, PK, LG, IM, and VS are consultants for AppliedVR, Inc. CC is a contractor for AppliedVR, Inc. BD is the chief science advisor for AppliedVR, Inc. BD has authored or coauthored 5 pain treatment books for patients and clinicians, and receives royalties for 4 books. BD is the principal investigator for pain research grants and awards from the National Institutes of Health (NIH) and the Patient-Centered Research Outcomes Research Institute (none specific to the current work). BD is a co-investigator on 2 NIH research grants investigating virtual reality analgesia; neither of these grants is specific to the current work. BD serves on the Board of Directors for the American Academy of Pain Medicine and is on the Board of Directors for the Institute for Brain Potential. BD is a scientific member of the NIH Interagency Pain Research Coordinating Committee, the Centers for Disease Control and Prevention (CDC) Opioid Workgroup (2020-2021), and the Pain Advisory Group of the American Psychological Association.
Figures


References
-
- Spiegel B, Fuller G, Lopez M, Dupuy T, Noah B, Howard A, Albert M, Tashjian V, Lam R, Ahn J, Dailey F, Rosen BT, Vrahas M, Little M, Garlich J, Dzubur E, IsHak W, Danovitch I. Virtual reality for management of pain in hospitalized patients: A randomized comparative effectiveness trial. PLoS One. 2019;14(8):e0219115. doi: 10.1371/journal.pone.0219115. https://dx.plos.org/10.1371/journal.pone.0219115 PONE-D-18-32917 - DOI - PMC - PubMed
-
- Atzori B, Hoffman HG, Vagnoli L, Patterson DR, Alhalabi W, Messeri A, Lauro Grotto R. Virtual Reality Analgesia During Venipuncture in Pediatric Patients With Onco-Hematological Diseases. Front Psychol. 2018 Dec 20;9:2508. doi: 10.3389/fpsyg.2018.02508. doi: 10.3389/fpsyg.2018.02508. - DOI - PMC - PubMed
-
- Darnall BD, Krishnamurthy P, Tsuei J, Minor JD. Self-Administered Skills-Based Virtual Reality Intervention for Chronic Pain: Randomized Controlled Pilot Study. JMIR Form Res. 2020 Jul 07;4(7):e17293. doi: 10.2196/17293. https://formative.jmir.org/2020/7/e17293/ v4i7e17293 - DOI - PMC - PubMed
-
- Garcia LM, Birckhead BJ, Krishnamurthy P, Sackman J, Mackey IG, Louis RG, Salmasi V, Maddox T, Darnall BD. An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19. J Med Internet Res. 2021 Feb 22;23(2):e26292. doi: 10.2196/26292. https://www.jmir.org/2021/2/e26292/ v23i2e26292 - DOI - PMC - PubMed
-
- Brea-Gómez B, Torres-Sánchez I, Ortiz-Rubio A, Calvache-Mateo A, Cabrera-Martos I, López-López L, Valenza MC. Virtual Reality in the Treatment of Adults with Chronic Low Back Pain: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int J Environ Res Public Health. 2021 Nov 11;18(22):11806. doi: 10.3390/ijerph182211806. https://www.mdpi.com/resolver?pii=ijerph182211806 ijerph182211806 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

