Lenvatinib plus pembrolizumab in Japanese patients with endometrial cancer: Results from Study 309/KEYNOTE-775
- PMID: 35612971
- PMCID: PMC9530883
- DOI: 10.1111/cas.15436
Lenvatinib plus pembrolizumab in Japanese patients with endometrial cancer: Results from Study 309/KEYNOTE-775
Abstract
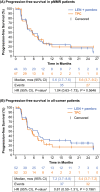
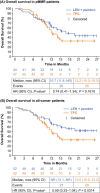
Study 309/KEYNOTE-775 is a phase 3 open-label, randomized trial of lenvatinib plus pembrolizumab versus treatment of physician's choice (TPC) in patients with advanced endometrial cancer with progression after platinum-based therapy. Primary endpoints of superiority for lenvatinib plus pembrolizumab were met for progression-free survival (PFS) and overall survival (OS) in all-comers (ie, regardless of mismatch repair [MMR] status) and patients with MMR proficiency (pMMR). We present results for the Japanese subset. Patients were randomized to oral lenvatinib 20 mg/day plus intravenous pembrolizumab 200 mg every 3 weeks (Q3W; up to 35 cycles of pembrolizumab) or TPC (intravenous doxorubicin 60 mg/m2 Q3W or paclitaxel 80 mg/m2 QW [3 weeks on/1 week off]). Primary endpoints were PFS by blinded independent central review per RECIST version 1.1 and OS. One hundred four patients were randomized in Japan (data cutoff, October 26, 2020; median follow-up, 11.8 [range, 1.1-26.9] months). Hazard ratios (HRs) for PFS with lenvatinib plus pembrolizumab versus TPC were 1.04 (95% CI, 0.63-1.73) in patients with pMMR and 0.81 (0.50-1.31) in all-comers. Hazard ratios for OS were 0.74 (0.41-1.34) with pMMR and 0.59 (0.33-1.04) for all-comers. Adverse events were manageable and led to discontinuation of one/both study drugs in 36.5% of patients in the lenvatinib plus pembrolizumab group versus 7.8% in the TPC group. Similar to the global Study 309/KEYNOTE-775 results, this analysis suggested favorable efficacy and manageable safety with lenvatinib plus pembrolizumab after platinum-based chemotherapy in Japanese patients with advanced endometrial cancer and supports this combination as a new standard of care in this population.
Keywords: Japan; endometrial cancer; lenvatinib; pembrolizumab; treatment outcomes.
© 2022 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- Center for Cancer Control and Information Services NCC . Monitoring of cancer incidence in Japan ‐ Survival 2009–2011 report 2020. 2020.
-
- Merck's KEYTRUDA® (pembrolizumab) receives five new approvals in Japan, including in advanced non‐small cell lung cancer, as adjuvant therapy for melanoma, and in advanced microsatellite instability‐high tumors. 2019. https://www.merck.com/news/mercks‐keytruda‐pembrolizumab‐receives‐five‐n.... Accessed October 18, 2021.
-
- LENVIMA® (lenvatinib) . Full Prescribing Information. Eisai Inc.; 2020.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

