The translational challenge in Chagas disease drug development
- PMID: 35613156
- PMCID: PMC9128742
- DOI: 10.1590/0074-02760200501
The translational challenge in Chagas disease drug development
Abstract
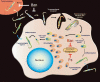
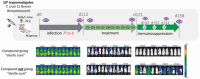
Chagas disease is a neglected tropical disease caused by the protozoan parasite Trypanosoma cruzi. There is an urgent need for safe, effective, and accessible new treatments since the currently approved drugs have serious limitations. Drug development for Chagas disease has historically been hampered by the complexity of the disease, critical knowledge gaps, and lack of coordinated R&D efforts. This review covers some of the translational challenges associated with the progression of new chemical entities from preclinical to clinical phases of development, and discusses how recent technological advances might allow the research community to answer key questions relevant to the disease and to overcome hurdles in R&D for Chagas disease.
Figures


Comment in
-
Chagas disease treatment: a 120-year-old challenge to public health.Mem Inst Oswaldo Cruz. 2022 May 23;117:e210501chgsa. doi: 10.1590/0074-02760210501chgsa. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35613159 Free PMC article. No abstract available.
-
Comment on "The translational challenge in Chagas disease drug development" by Kratz et al.Mem Inst Oswaldo Cruz. 2022 May 23;117:e210501chgsb. doi: 10.1590/0074-02760210501chgsb. eCollection 2022. Mem Inst Oswaldo Cruz. 2022. PMID: 35613160 Free PMC article. No abstract available.
References
-
- WHO WHO fact sheet No 340. 2020. http://www.who.int/mediacentre/factsheets/fs340/en/
-
- Pérez-Molina JA, Molina I. Chagas disease. Lancet. 2018;391(10115):82–94. - PubMed
-
- WHO Fourth WHO report on neglected tropical diseases: integrating neglected tropical diseases in global health and development. 2020. https://www.who.int/neglected_diseases/resources/9789241565448/en/
-
- Sosa-Estani S, Segura EL. Etiological treatment in patients infected by Trypanosoma cruzi experiences in Argentina. Curr Opin Infect Dis. 2006;19(6):583–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

