Actin-binding protein filamin-A drives tau aggregation and contributes to progressive supranuclear palsy pathology
- PMID: 35613261
- PMCID: PMC9132466
- DOI: 10.1126/sciadv.abm5029
Actin-binding protein filamin-A drives tau aggregation and contributes to progressive supranuclear palsy pathology
Abstract
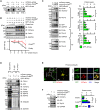
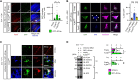
While amyloid-β lies upstream of tau pathology in Alzheimer's disease, key drivers for other tauopathies, including progressive supranuclear palsy (PSP), are largely unknown. Various tau mutations are known to facilitate tau aggregation, but how the nonmutated tau, which most cases with PSP share, increases its propensity to aggregate in neurons and glial cells has remained elusive. Here, we identified genetic variations and protein abundance of filamin-A in the PSP brains without tau mutations. We provided in vivo biochemical evidence that increased filamin-A levels enhance the phosphorylation and insolubility of tau through interacting actin filaments. In addition, reduction of filamin-A corrected aberrant tau levels in the culture cells from PSP cases. Moreover, transgenic mice carrying human filamin-A recapitulated tau pathology in the neurons. Our data highlight that filamin-A promotes tau aggregation, providing a potential mechanism by which filamin-A contributes to PSP pathology.
Figures




References
-
- Uttl B., Santacruz P., Litvan I., Grafman J., Caregiving in progressive supranuclear palsy. Neurology 51, 1303–1309 (1998). - PubMed
-
- Glasmacher S. A., Leigh P. N., Saha R. A., Predictors of survival in progressive supranuclear palsy and multiple system atrophy: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 88, 402–411 (2017). - PubMed
-
- Yoshida M., Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 34, 555–570 (2014). - PubMed
-
- Ishigaki S., Fujioka Y., Okada Y., Riku Y., Udagawa T., Honda D., Yokoi S., Endo K., Ikenaka K., Takagi S., Iguchi Y., Sahara N., Takashima A., Okano H., Yoshida M., Warita H., Aoki M., Watanabe H., Okado H., Katsuno M., Sobue G., Altered tau isoform ratio caused by loss of FUS and SFPQ function leads to FTLD-like phenotypes. Cell Rep. 18, 1118–1131 (2017). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

