Phase 2a randomised controlled feasibility trial of a new 'balanced binocular viewing' treatment for unilateral amblyopia in children age 3-8 years: trial protocol
- PMID: 35613759
- PMCID: PMC9131062
- DOI: 10.1136/bmjopen-2021-051423
Phase 2a randomised controlled feasibility trial of a new 'balanced binocular viewing' treatment for unilateral amblyopia in children age 3-8 years: trial protocol
Abstract
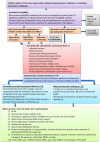
Introduction: Treatments for amblyopia, the most common vision deficit in children, often have suboptimal results. Occlusion/atropine blurring are fraught with poor adherence, regression and recurrence. These interventions target only the amblyopic eye, failing to address imbalances of cortical input from the two eyes ('suppression'). Dichoptic treatments manipulate binocular visual experience to rebalance input. Poor adherence in early trials of dichoptic therapies inspired our development of balanced binocular viewing (BBV), using movies as child-friendly viewable content. Small observational studies indicate good adherence and efficacy. A feasibility trial is needed to further test safety and gather information to design a full trial.
Methods/analysis: We will carry out an observer-masked parallel-group phase 2a feasibility randomised controlled trial at two sites, randomising 44 children aged 3-8 years with unilateral amblyopia to either BBV or standard occlusion/atropine blurring, with 1:1 allocation ratio. We will assess visual function at baseline, 8 and 16 weeks. The primary outcome is intervention safety at 16 weeks, measured as change in interocular suppression, considered to precede the onset of potential diplopia. Secondary outcomes include safety at other time points, eligibility, recruitment/retention rates, adherence, clinical outcomes. We will summarise baseline characteristics for each group and assess the treatment effect using analysis of covariance. We will compare continuous clinical secondary endpoints between arms using linear mixed effect models, and report feasibility endpoints using descriptive statistics.
Ethics/dissemination: This trial has been approved by the London-Brighton & Sussex Research Ethics Committee (18/LO/1204), National Health Service Health Research Authority and Medicines and Healthcare products Regulatory Agency. A lay advisory group will be involved with advising on and disseminating the results to non-professional audiences, including on websites of funder/participating institutions and inputting on healthcare professional audience children would like us to reach. Reporting to clinicians and scientists will be via internal and external meetings/conferences and peer-reviewed journals.
Trial registration number: NCT03754153.
Keywords: OPHTHALMOLOGY; Paediatric ophthalmology; Strabismus.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: AHD-N: Medical advisor for Santen, Novartis, CooperVision, SightGlassVision.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical