Reporting quality of clinical trial protocols: a repeated cross-sectional study about the Adherence to SPIrit Recommendations in Switzerland, CAnada and GErmany (ASPIRE-SCAGE)
- PMID: 35613804
- PMCID: PMC9125701
- DOI: 10.1136/bmjopen-2021-053417
Reporting quality of clinical trial protocols: a repeated cross-sectional study about the Adherence to SPIrit Recommendations in Switzerland, CAnada and GErmany (ASPIRE-SCAGE)
Abstract
Objectives: Comprehensive protocols are key for the planning and conduct of randomised clinical trials (RCTs). Evidence of low reporting quality of RCT protocols led to the publication of the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) checklist in 2013. We aimed to examine the quality of reporting of RCT protocols from three countries before and after the publication of the SPIRIT checklist.
Design: Repeated cross sectional study.
Setting: Swiss, German and Canadian research ethics committees (RECs).
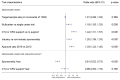
Participants: RCT protocols approved by RECs in 2012 (n=257) and 2016 (n=292).
Primary and secondary outcome measures: The primary outcomes were the proportion of reported SPIRIT items per protocol and the proportion of trial protocols reporting individual SPIRIT items. We compared these outcomes in protocols approved in 2012 and 2016, and built regression models to explore factors associated with adherence to SPIRIT. For each protocol, we also extracted information on general trial characteristics and assessed whether individual SPIRIT items were reported RESULTS: The median proportion of reported SPIRIT items among RCT protocols showed a non-significant increase from 72% (IQR, 63%-79%) in 2012 to 77% (IQR, 68%-82%) in 2016. However, in a preplanned subgroup analysis, we detected a significant improvement in investigator-sponsored protocols: the median proportion increased from 64% (IQR, 55%-72%) in 2012 to 76% (IQR, 64%-83%) in 2016, while for industry-sponsored protocols median adherence was 77% (IQR 72%-80%) for both years. The following trial characteristics were independently associated with lower adherence to SPIRIT: single-centre trial, no support from a clinical trials unit or contract research organisation, and investigator-sponsorship.
Conclusions: In 2012, industry-sponsored RCT protocols were reported more comprehensively than investigator-sponsored protocols. After publication of the SPIRIT checklist, investigator-sponsored protocols improved to the level of industry-sponsored protocols, which did not improve.
Keywords: Clinical trials; EPIDEMIOLOGY; Protocols & guidelines.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BvN is currently employed by Roche Pharma AG, Grenzach-Wyhlen, Germany. BK is currently employed by iOMEDICO AG, Freiburg, Germany. All other authors declare no financial relationships with any organisation that might have an interest in the submitted work and no other relationships or activities that could appear to have influenced the submitted work.
Figures

References
-
- World Medical Association . WMA Declaration of Helsinki - ethical principles for medical research involving human subjects, 2013. Available: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-pr... - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
