Sintilimab combined with bevacizumab in relapsed/persistent ovarian clear cell carcinoma (INOVA): an investigator-initiated, multicentre clinical trial-a study protocol of clinical trial
- PMID: 35613822
- PMCID: PMC9131073
- DOI: 10.1136/bmjopen-2021-058132
Sintilimab combined with bevacizumab in relapsed/persistent ovarian clear cell carcinoma (INOVA): an investigator-initiated, multicentre clinical trial-a study protocol of clinical trial
Abstract
Background: Ovarian clear cell carcinoma (OCCC) has an abysmal prognosis with a median overall survival (OS) of 25.3 months because of a low response to chemotherapy. The 5-year disease-specific survival rate after recurrence is 13.2%, with more than two-thirds of the patients dying within a year. Therefore, it is urgent to explore new therapeutic options for OCCC. Based on the characteristic immune-suppressive tumour microenvironment derived from the gene expression profile of OCCC, the combination of immunoantiangiogenesis therapy might have certain efficacy in recurrent/persistent OCCC. This trial aims to evaluate the efficacy and safety of sintilimab and bevacizumab in patients who have failed platinum-containing chemotherapy with recurrent or persistent OCCC.
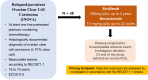
Method and analysis: In this multicentre, single-arm, open-label, investigator-initiated clinical trial, 38 patients will be assigned to receive sintilimab 200 mg plus bevacizumab 15 mg/kg every 3 weeks. The eligibility criteria include histologically diagnosed patients with recurrent or persistent OCCC who have been previously treated with at least one-line platinum-containing chemotherapy; patients with Eastern Cooperative Oncology Group (ECOG) performance status 0-2 with an expected survival greater than 12 weeks. The exclusion criteria include patients previously treated with immune checkpoint inhibitor and patients with contraindications of bevacizumab and sintilimab. The primary endpoint is the objective response rate. The secondary endpoints are progression-free survival, time to response, duration of response, disease control rate, OS, safety and quality of life. Statistical significance was defined as p<0.05.
Ethics and dissemination: This trial was approved by the Research Ethics Commission of Tongji Medical College of Huazhong University of Science and Technology (2020-S337). The protocol of this study is registered at www.
Clinicaltrials: gov. The trial results will be published in peer-reviewed journals and at conferences.
Trial registration number: NCT04735861; Clinicaltrials. gov.
Keywords: gynaecological oncology; immunology; therapeutics.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- The International Agency for Research on Cancer . Global cancer statistics, 2021. Available: https://gcoiarcfr/ [Accessed Mar 2021].
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
