Role of Sleep in Formation of Relational Associative Memory
- PMID: 35613890
- PMCID: PMC9270916
- DOI: 10.1523/JNEUROSCI.2044-21.2022
Role of Sleep in Formation of Relational Associative Memory
Abstract
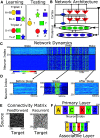
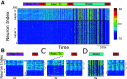
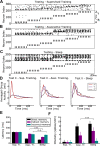
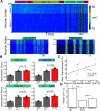
Relational memory, the ability to make and remember associations between objects, is an essential component of mammalian reasoning. In relational memory tasks, it has been shown that periods of offline processing, such as sleep, are critical to making indirect associations. To understand biophysical mechanisms behind the role of sleep in improving relational memory, we developed a model of the thalamocortical network to test how slow-wave sleep affects performance on an unordered relational memory task. First, the model was trained in the awake state on a paired associate inference task, in which the model learned to recall direct associations. After a period of subsequent slow-wave sleep, the model developed the ability to recall indirect associations. We found that replay, during sleep, of memory patterns learned in awake increased synaptic connectivity between neurons representing the item that was overlapping between tasks and neurons representing the unlinked items of the different tasks; this forms an attractor that enables indirect memory recall. Our study predicts that overlapping items between indirectly associated tasks are essential for relational memory, and sleep can reactivate pathways to and from overlapping items to the unlinked objects to strengthen these pathways and form new relational memories.SIGNIFICANCE STATEMENT Experimental studies have shown that some types of associative memory, such as transitive inference and relational memory, can improve after sleep. Still, it remains unknown what specific mechanisms are responsible for these sleep-related changes. In this new work, we addressed this problem by building a thalamocortical network model that can learn relational memory tasks and that can be simulated in awake or sleep states. We found that memory traces learned in awake were replayed during slow waves of NREM sleep and revealed that replay increased connections to and from overlapping memory items to form new relational memories. Our work discovered specific mechanisms behind the role of sleep in associative memory and made testable predictions about how sleep augments associative learning.
Keywords: learning and memory; memory consolidation; relational memory; sleep; synaptic plasticity; transitive inference.
Copyright © 2022 the authors.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
