Improved trends in survival and engraftment after single cord blood transplantation for adult acute myeloid leukemia
- PMID: 35614057
- PMCID: PMC9132934
- DOI: 10.1038/s41408-022-00678-6
Improved trends in survival and engraftment after single cord blood transplantation for adult acute myeloid leukemia
Abstract
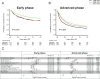
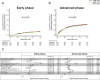
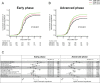
Unrelated cord blood transplantation (CBT) is an alternative curative option for adult patients with acute myeloid leukemia (AML) who need allogeneic hematopoietic cell transplantation (HCT) but lack an HLA-matched related or unrelated donor. However, large-scale data are lacking on CBT outcomes for unselected adult AML. To investigate the trends of survival and engraftment after CBT over the past 22 years, we retrospectively evaluated the data of patients with AML in Japan according to the time period of CBT (1998-2007 vs 2008-2013 vs 2014-2019). A total of 5504 patients who received single-unit CBT as first allogeneic HCT for AML were included. Overall survival (OS) at 2 years significantly improved over time. The improved OS among patients in ≥ complete remission (CR)3 and active disease at CBT was mainly due to a reduction of relapse-related mortality, whereas among patients in first or second CR at CBT, this was due mainly to a reduction of non-relapse mortality. The trends of neutrophil engraftment also improved over time. This experience demonstrated that the survival and engraftment rate after CBT for this group has improved over the past 22 years.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rocha V, Gluckman E, Eurocord-Netcord registry and European Blood and Marrow Transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br J Haematol. 2009;147:262–74. doi: 10.1111/j.1365-2141.2009.07883.x. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

