Cell type-specific biotin labeling in vivo resolves regional neuronal and astrocyte proteomic differences in mouse brain
- PMID: 35614064
- PMCID: PMC9132937
- DOI: 10.1038/s41467-022-30623-x
Cell type-specific biotin labeling in vivo resolves regional neuronal and astrocyte proteomic differences in mouse brain
Abstract
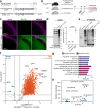
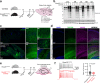
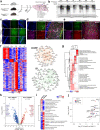
Proteomic profiling of brain cell types using isolation-based strategies pose limitations in resolving cellular phenotypes representative of their native state. We describe a mouse line for cell type-specific expression of biotin ligase TurboID, for in vivo biotinylation of proteins. Using adenoviral and transgenic approaches to label neurons, we show robust protein biotinylation in neuronal soma and axons throughout the brain, allowing quantitation of over 2000 neuron-derived proteins spanning synaptic proteins, transporters, ion channels and disease-relevant druggable targets. Next, we contrast Camk2a-neuron and Aldh1l1-astrocyte proteomes and identify brain region-specific proteomic differences within both cell types, some of which might potentially underlie the selective vulnerability to neurological diseases. Leveraging the cellular specificity of proteomic labeling, we apply an antibody-based approach to uncover differences in neuron and astrocyte-derived signaling phospho-proteins and cytokines. This approach will facilitate the characterization of cell-type specific proteomes in a diverse number of tissues under both physiological and pathological states.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- F31 AG071319/AG/NIA NIH HHS/United States
- U54 AG065187/AG/NIA NIH HHS/United States
- P30 AG066511/AG/NIA NIH HHS/United States
- F32 AG064862/AG/NIA NIH HHS/United States
- P50 AG025688/AG/NIA NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- K08 NS099474/NS/NINDS NIH HHS/United States
- R56 AG072473/AG/NIA NIH HHS/United States
- R01 AG061800/AG/NIA NIH HHS/United States
- RF1 AG062181/AG/NIA NIH HHS/United States
- RF1 AG071587/AG/NIA NIH HHS/United States
- R01 NS115994/NS/NINDS NIH HHS/United States
- R01 NS114130/NS/NINDS NIH HHS/United States
- U01 AG061357/AG/NIA NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- R01 AG075820/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

