Identification and validation of a novel pyroptosis-related lncRNAs signature associated with prognosis and immune regulation of hepatocellular carcinoma
- PMID: 35614201
- PMCID: PMC9133103
- DOI: 10.1038/s41598-022-13046-y
Identification and validation of a novel pyroptosis-related lncRNAs signature associated with prognosis and immune regulation of hepatocellular carcinoma
Abstract
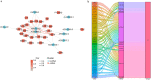
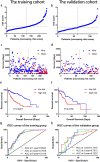
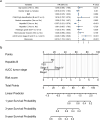
Pyroptosis is an inflammatory form of cell death triggered by certain inflammasomes. However, research concerning pyroptosis-related lncRNAs in hepatocellular carcinoma (HCC) remains scarce. This study aims to explore the prognostic pyroptosis-related long non-coding RNAs (lncRNAs) of HCC patients. Data of 373 HCC patients were obtained from the TCGA database. The entire cohort was randomly divided into a training cohort and a validation cohort in a 2:1 ratio. Pyroptosis-related lncRNAs were identified by the Pearson correlation analysis with reported pyroptosis-related genes. LASSO Cox regression was used to construct the signature. A prognostic signature consisting of nine pyroptosis-related lncRNAs was identified, and patients with lower risk scores had a better prognosis than those with higher risk scores. Multivariate Cox regression analysis showed that the signature was an independent risk factor for prognosis in both the training and validation cohorts. In the training cohort, the area under the signature curve reached 0.8043 at 1-year, 0.7878 at 2-year, and 0.8118 at 3-year; in the validation cohort, it reached 0.7315 at 1-year, 0.7372 at 2-year, and 0.7222 at 3-year. Gene set enrichment analysis (GSEA) suggested associations between the signature and several immune-related pathways. The expression of multiple immune checkpoints was also increased in the high-risk group, including PD-1, PD-L1, CTLA4, B7-H3, VSIR, LAG3, and TIGIT. A novel pyroptosis-related lncRNA signature, which may be associated with tumor immunity and potentially serve as an indicator for immunotherapy, has been identified to precisely predict the prognosis of HCC patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

