Neuropathic pain caused by miswiring and abnormal end organ targeting
- PMID: 35614217
- PMCID: PMC9159955
- DOI: 10.1038/s41586-022-04777-z
Neuropathic pain caused by miswiring and abnormal end organ targeting
Abstract
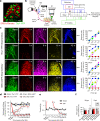
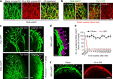
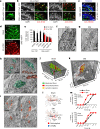
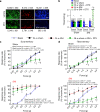
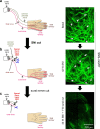
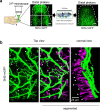
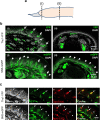
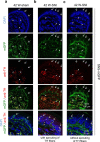
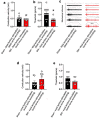
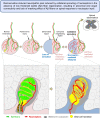
Nerve injury leads to chronic pain and exaggerated sensitivity to gentle touch (allodynia) as well as a loss of sensation in the areas in which injured and non-injured nerves come together1-3. The mechanisms that disambiguate these mixed and paradoxical symptoms are unknown. Here we longitudinally and non-invasively imaged genetically labelled populations of fibres that sense noxious stimuli (nociceptors) and gentle touch (low-threshold afferents) peripherally in the skin for longer than 10 months after nerve injury, while simultaneously tracking pain-related behaviour in the same mice. Fully denervated areas of skin initially lost sensation, gradually recovered normal sensitivity and developed marked allodynia and aversion to gentle touch several months after injury. This reinnervation-induced neuropathic pain involved nociceptors that sprouted into denervated territories precisely reproducing the initial pattern of innervation, were guided by blood vessels and showed irregular terminal connectivity in the skin and lowered activation thresholds mimicking low-threshold afferents. By contrast, low-threshold afferents-which normally mediate touch sensation as well as allodynia in intact nerve territories after injury4-7-did not reinnervate, leading to an aberrant innervation of tactile end organs such as Meissner corpuscles with nociceptors alone. Genetic ablation of nociceptors fully abrogated reinnervation allodynia. Our results thus reveal the emergence of a form of chronic neuropathic pain that is driven by structural plasticity, abnormal terminal connectivity and malfunction of nociceptors during reinnervation, and provide a mechanistic framework for the paradoxical sensory manifestations that are observed clinically and can impose a heavy burden on patients.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures









Comment in
-
Nerve regrowth can be painful.Nature. 2022 Jun;606(7912):32-33. doi: 10.1038/d41586-022-01243-8. Nature. 2022. PMID: 35614254 No abstract available.
References
-
- Devor, M. in Wall and Melzack’s Textbook of Pain 5th edn (eds McMahon, S. B. & Koltzenburg, M.) 905–927 (Churchill Livingstone, 2006).
-
- Finnerup NB, Kuner R, Jensen TS. Neuropathic pain: from mechanisms to treatment. Physiol. Rev. 2021;101:259–301. - PubMed
-
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

