Developmental dynamics of two bipotent thymic epithelial progenitor types
- PMID: 35614226
- PMCID: PMC9159946
- DOI: 10.1038/s41586-022-04752-8
Developmental dynamics of two bipotent thymic epithelial progenitor types
Erratum in
-
Publisher Correction: Developmental dynamics of two bipotent thymic epithelial progenitor types.Nature. 2024 Feb;626(8000):E17. doi: 10.1038/s41586-024-07129-1. Nature. 2024. PMID: 38302744 Free PMC article. No abstract available.
Abstract
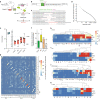
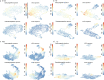
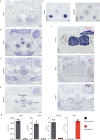
T cell development in the thymus is essential for cellular immunity and depends on the organotypic thymic epithelial microenvironment. In comparison with other organs, the size and cellular composition of the thymus are unusually dynamic, as exemplified by rapid growth and high T cell output during early stages of development, followed by a gradual loss of functional thymic epithelial cells and diminished naive T cell production with age1-10. Single-cell RNA sequencing (scRNA-seq) has uncovered an unexpected heterogeneity of cell types in the thymic epithelium of young and aged adult mice11-18; however, the identities and developmental dynamics of putative pre- and postnatal epithelial progenitors have remained unresolved1,12,16,17,19-27. Here we combine scRNA-seq and a new CRISPR-Cas9-based cellular barcoding system in mice to determine qualitative and quantitative changes in the thymic epithelium over time. This dual approach enabled us to identify two principal progenitor populations: an early bipotent progenitor type biased towards cortical epithelium and a postnatal bipotent progenitor population biased towards medullary epithelium. We further demonstrate that continuous autocrine provision of Fgf7 leads to sustained expansion of thymic microenvironments without exhausting the epithelial progenitor pools, suggesting a strategy to modulate the extent of thymopoietic activity.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures













References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

