Long COVID after breakthrough SARS-CoV-2 infection
- PMID: 35614233
- PMCID: PMC9307472
- DOI: 10.1038/s41591-022-01840-0
Long COVID after breakthrough SARS-CoV-2 infection
Abstract
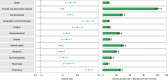
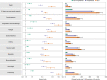
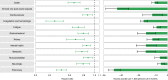
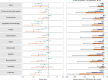
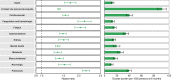
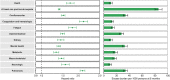
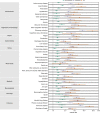
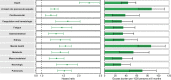
The post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection-also referred to as Long COVID-have been described, but whether breakthrough SARS-CoV-2 infection (BTI) in vaccinated people results in post-acute sequelae is not clear. In this study, we used the US Department of Veterans Affairs national healthcare databases to build a cohort of 33,940 individuals with BTI and several controls of people without evidence of SARS-CoV-2 infection, including contemporary (n = 4,983,491), historical (n = 5,785,273) and vaccinated (n = 2,566,369) controls. At 6 months after infection, we show that, beyond the first 30 days of illness, compared to contemporary controls, people with BTI exhibited a higher risk of death (hazard ratio (HR) = 1.75, 95% confidence interval (CI): 1.59, 1.93) and incident post-acute sequelae (HR = 1.50, 95% CI: 1.46, 1.54), including cardiovascular, coagulation and hematologic, gastrointestinal, kidney, mental health, metabolic, musculoskeletal and neurologic disorders. The results were consistent in comparisons versus the historical and vaccinated controls. Compared to people with SARS-CoV-2 infection who were not previously vaccinated (n = 113,474), people with BTI exhibited lower risks of death (HR = 0.66, 95% CI: 0.58, 0.74) and incident post-acute sequelae (HR = 0.85, 95% CI: 0.82, 0.89). Altogether, the findings suggest that vaccination before infection confers only partial protection in the post-acute phase of the disease; hence, reliance on it as a sole mitigation strategy may not optimally reduce long-term health consequences of SARS-CoV-2 infection. The findings emphasize the need for continued optimization of strategies for primary prevention of BTI and will guide development of post-acute care pathways for people with BTI.
© 2022. This is a U.S. government work and not under copyright protection in the U.S.; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
How common is long COVID? Why studies give different answers.Nature. 2022 Jun;606(7916):852-853. doi: 10.1038/d41586-022-01702-2. Nature. 2022. PMID: 35725828 No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

