A longitudinal study of the arterio-venous fistula maturation of a single patient over 15 weeks
- PMID: 35614372
- PMCID: PMC9283179
- DOI: 10.1007/s10237-022-01586-1
A longitudinal study of the arterio-venous fistula maturation of a single patient over 15 weeks
Abstract
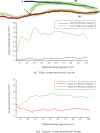
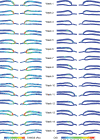
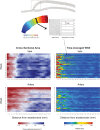
Arterio-venous fistula creation is the preferred vascular access for haemodialysis, but has a large failure rate in the maturation period. Previous research, considering the remodelling mechanisms for failure-to-mature patients, has been limited by obtaining the patient-specific boundary conditions at only a few points in the patient history. Here, a non-invasive imaging system was used to reconstruct the three-dimensional vasculature, and computational fluid dynamics was used to analyse the haemodynamics for one patient over 15 weeks. The analysis suggested evidence of a control mechanism, which adjusts the lumen diameter to keep the wall shear stress near constant in the proximal regions of the vein and artery. Additionally, the vein and artery were shown to remodel at different growth rates, and the blood flow rate also saw the largest increase within the first week. Wall shear stress at time of creation may be a useful indicator for successful AVF maturation.
Keywords: Arterio-venous fistula; Computational fluid dynamics; Dialysis; Vascular access.
© 2022. Crown.
Conflict of interest statement
The authors have no disclosures. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000 (5). Informed consent was obtained from all patients for being included in the study.
Figures








References
MeSH terms
LinkOut - more resources
Full Text Sources

