Accuracy and tolerability of self-sampling of capillary blood for analysis of inflammation and autoantibodies in rheumatoid arthritis patients-results from a randomized controlled trial
- PMID: 35614488
- PMCID: PMC9130452
- DOI: 10.1186/s13075-022-02809-7
Accuracy and tolerability of self-sampling of capillary blood for analysis of inflammation and autoantibodies in rheumatoid arthritis patients-results from a randomized controlled trial
Abstract
Background: Rheumatoid arthritis (RA) requires early diagnosis and tight surveillance of disease activity. Remote self-collection of blood for the analysis of inflammation markers and autoantibodies could improve the monitoring of RA and facilitate the identification of individuals at-risk for RA.
Objective: Randomized, controlled trial to evaluate the accuracy, feasibility, and acceptability of an upper arm self-sampling device (UA) and finger prick-test (FP) to measure capillary blood from RA patients for C-reactive protein (CRP) levels and the presence of IgM rheumatoid factor (RF IgM) and anti-cyclic citrullinated protein antibodies (anti-CCP IgG).
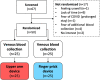
Methods: RA patients were randomly assigned in a 1:1 ratio to self-collection of capillary blood via UA or FP. Venous blood sampling (VBS) was performed as a gold standard in both groups to assess the concordance of CRP levels as well as RF IgM and CCP IgG. General acceptability and pain during sampling were measured and compared between UA, FP, and VBS. The number of attempts for successful sampling, requests for assistance, volume, and duration of sample collection were also assessed.
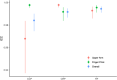
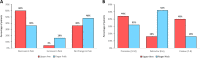
Results: Fifty seropositive RA patients were included. 49/50 (98%) patients were able to successfully collect capillary blood. The overall agreement between capillary and venous analyses for CRP (0.992), CCP IgG (0.984), and RF IgM (0.994) were good. In both groups, 4/25 (16%) needed a second attempt and 8/25 (32%) in the UA and 7/25 (28%) in the FP group requested assistance. Mean pain scores for capillary self-sampling (1.7/10 ± 1.1 (UA) and 1.9/10 ± 1.9 (FP)) were significantly lower on a numeric rating scale compared to venous blood collection (UA: 2.8/10 ± 1.7; FP: 2.1 ± 2.0) (p=0.003). UA patients were more likely to promote the use of capillary blood sampling (net promoter score: +28% vs. -20% for FP) and were more willing to perform blood collection at home (60% vs. 32% for FP).
Conclusions: These data show that self-sampling is accurate and feasible within one attempt by the majority of patients without assistance, allowing tight monitoring of RA disease activity as well as identifying individuals at-risk for RA. RA patients seem to prefer upper arm-based self-sampling to traditional finger pricking.
Trial registration: DRKS.de Identifier: DRKS00023526 . Registered on November 6, 2020.
Keywords: Capillary blood; Disease activity; Rheumatoid arthritis; Self-sampling.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Dejaco C, Alunno A, Bijlsma JW, Boonen A, Combe B, Finckh A, et al. Influence of COVID-19 pandemic on decisions for the management of people with inflammatory rheumatic and musculoskeletal diseases: a survey among EULAR countries. Ann Rheum Dis. 2020;annrheumdis-2020-218697. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

