Delineating the conformational landscape and intrinsic properties of the angiotensin II type 2 receptor using a computational study
- PMID: 35615027
- PMCID: PMC9117689
- DOI: 10.1016/j.csbj.2022.05.012
Delineating the conformational landscape and intrinsic properties of the angiotensin II type 2 receptor using a computational study
Abstract
As a key regulator for the renin-angiotensin system, a class A G protein-coupled receptor (GPCR), AngII type 2 receptor (AT2R), plays a pivotal role in the homeostasis of the cardiovascular system. Compared with other GPCRs, AT2R has a unique antagonist-bound conformation and its mechanism is still an enigma. Here, we applied combined dynamic and evolutional approaches to investigate the conformational space and intrinsic properties of AT2R. With molecular dynamic simulations, Markov State Models, and statistics coupled analysis, we captured the conformational landscape of AT2R and identified its uniquity from both dynamical and evolutional viewpoints. A cryptic pocket was also discovered in the intermediate state during conformation transitions. These findings offer a deeper understanding of the AT2R mechanism at an atomic level and provide hints for the design of novel AT2R modulators.
Keywords: AT2R; Conformational dynamics; Cryptic pocket; G protein-coupled receptors; MD simulations.
© 2022 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


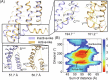



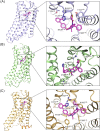

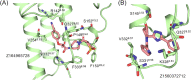
References
-
- Mehta P.K., Griendling K.K. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:82–97. - PubMed
-
- Zaman M.A., Oparil S., Calhoun D.A. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 2002;1:621–636. - PubMed
-
- de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
