Intravitreal Anti-vascular Endothelial Growth Factor Injection for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis
- PMID: 35615084
- PMCID: PMC9124790
- DOI: 10.3389/fmed.2022.884608
Intravitreal Anti-vascular Endothelial Growth Factor Injection for Retinopathy of Prematurity: A Systematic Review and Meta-Analysis
Abstract
Background: Laser photocoagulation and/or intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections constitute the current standard treatment for retinopathy of prematurity (ROP). This systematic review and meta-analysis aimed to assess the efficacy and safety of anti-VEGF monotherapy for ROP treatment using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach.
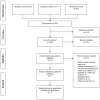
Methods: We searched the Medline, Embase, and Cochrane Central Register of Controlled Trials (CENTRAL) databases. We included randomized controlled trials (RCTs) that compared intravitreal anti-VEGF monotherapy (e.g., bevacizumab, ranibizumab, aflibercept, and pegaptanib) with laser photocoagulation in preterm infants with ROP. We evaluated the rates of recurrence, treatment switching, retreatment, adverse events, and mortality. The risk ratio (RR) was used to represent dichotomous outcomes. Data were pooled using the inverse variance weighting method. The quality of evidence was assessed using the GRADE approach. Risk of bias was assessed using the Revised Cochrane risk of bias tool for randomized trials.
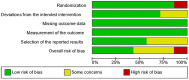
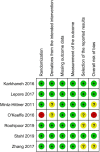
Results: Seven RCTs (n = 579; 1,158 eyes) were deemed eligible. Three RCTs had an overall low risk of bias, three had some concerns, and one had an overall high risk of bias. The pooled effect estimate showed a statistically significant reduction in adverse events in favor of anti-VEGF monotherapy [RR = 0.17, 95% confidence interval (CI) 0.07-0.44]. The pooled analysis showed no significant difference between the anti-VEGF and laser groups in terms of recurrence rate (RR = 1.56, 95% CI 0.23-10.54), treatment switching (RR = 2.92, 95% CI 0.40-21.05), retreatment (RR = 1.56, 95% CI 0.35-6.96), and mortality rate (RR = 1.28, 95% CI 0.48-3.41).
Conclusion: Overall, intravitreal anti-VEGF monotherapy was associated with fewer adverse events than laser therapy, rated as high quality of evidence according to the GRADE criteria. Pooled analysis revealed no significant difference between the two arms with respect to the recurrence rate, treatment switching, retreatment, and mortality rate, with quality of evidence ranging from moderate to very low as per the GRADE approach.
Systematic review registration: [https://www.crd.york.ac.uk/prospero/#recordDetails], identifier [CRD42021270077].
Keywords: anti-vascular endothelial growth factor; bevacizumab; laser photocoagulation; ranibizumab; retinopathy of prematurity.
Copyright © 2022 Taher, Ghaddaf, Al-Ghamdi, Homsi, Al-Harbi, Alomari and Almarzouki.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Dikci S, Ceylan OM, Demirel S, Yılmaz T. Which dose of bevacizumab is more effective for the treatment of aggressive posterior retinopathy of prematurity: lower or higher dose? Arq Bras Oftalmol. (2018) 81:12–7. - PubMed
-
- Popovic MM, Nichani P, Muni RH, Mireskandari K, Tehrani NN, Kertes PJ. Intravitreal antivascular endothelial growth factor injection versus laser photocoagulation for retinopathy of prematurity: a meta-analysis of 3,701 eyes. Surv Ophthalmol. (2021) 66:572–84. 10.1016/j.survophthal.2020.12.002 - DOI - PubMed
-
- The Royal College of Ophthalmologists. Consultation on Treating Retinopathy of Prematurity in the UK. London: The Royal College of Ophthalmologists; (2022).
Publication types
LinkOut - more resources
Full Text Sources

