Molecular Determinants of in vitro Plant Regeneration: Prospects for Enhanced Manipulation of Lettuce (Lactuca sativa L.)
- PMID: 35615120
- PMCID: PMC9125155
- DOI: 10.3389/fpls.2022.888425
Molecular Determinants of in vitro Plant Regeneration: Prospects for Enhanced Manipulation of Lettuce (Lactuca sativa L.)
Abstract
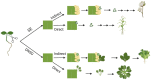
In vitro plant regeneration involves dedifferentiation and molecular reprogramming of cells in order to regenerate whole organs. Plant regeneration can occur via two pathways, de novo organogenesis and somatic embryogenesis. Both pathways involve intricate molecular mechanisms and crosstalk between auxin and cytokinin signaling. Molecular determinants of both pathways have been studied in detail in model species, but little is known about the molecular mechanisms controlling de novo shoot organogenesis in lettuce. This review provides a synopsis of our current knowledge on molecular determinants of de novo organogenesis and somatic embryogenesis with an emphasis on the former as well as provides insights into applying this information for enhanced in vitro regeneration in non-model species such as lettuce (Lactuca sativa L.).
Keywords: Lactuca sativa (L.); WUSCHEL; lettuce; organogenesis; regeneration; somatic embryogenesis.
Copyright © 2022 Bull and Michelmore.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Alconero R. (1983). Regeneration of plants from cell suspensions of Lactuca saligna, Lactuca sativa, and Lactuca serriola. HortScience 18 305–307.
-
- Ampomah-Dwamena C., Conner A. J., Fautrier A. G. (1997). Genotypic response of lettuce cotyledons to regeneration in vitro. Sci. Horticult. 71 137–145. 10.1016/s0304-4238(97)00098-8 - DOI
Publication types
LinkOut - more resources
Full Text Sources

