Efficacy of a third-generation oncolytic herpes simplex virus in refractory soft tissue sarcoma xenograft models
- PMID: 35615265
- PMCID: PMC9118137
- DOI: 10.1016/j.omto.2022.04.010
Efficacy of a third-generation oncolytic herpes simplex virus in refractory soft tissue sarcoma xenograft models
Abstract
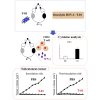
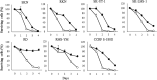
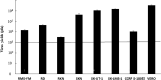
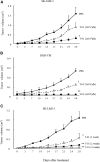
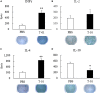
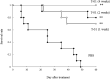
Malignant soft tissue tumors, particularly highly malignant leiomyosarcomas, are resistant to chemotherapy and associated with a poor prognosis. T-01, a third-generation genetically modified herpes simplex virus type 1, replicates in tumor cells alone and exerts a cell-killing effect. The current study aimed to investigate the antitumor effect of T-01, which is a novel treatment for leiomyosarcoma. In vitro, six human cell lines and one mouse sarcoma cell line were assessed for T-01 cytotoxicity. In vivo, the efficacy of T-01 was examined in subcutaneously transplanted leiomyosarcoma (SK-LMS-1) cells and subcutaneously or intraperitoneally transplanted mouse sarcoma (CCRF S-180II) cells. Cytokines were assessed using ELISpot assay with splenocytes from the allogeneic models for immunological evaluation. T-01 showed cytotoxicity in all seven cell lines (p < 0.001). In the SK-LMS-1 xenotransplantation model, tumor growth was suppressed by T-01 administration (p = 0.02). In the CCRF S-180II subcutaneous tumor model, bilateral tumor growth was significantly suppressed in the T-01-treated group compared with the control group (p < 0.001). In the peritoneal dissemination model, T-01 treatment caused significant survival prolongation compared with the control (p < 0.01). In conclusion, third-generation genetically modified herpes simplex virus type 1 may be an effective novel therapy against refractory sarcomas.
Keywords: T-01; herpes simplex virus; malignant soft tissue tumor; mouse xenograft model; oncolytic virus.
© 2022 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Efficacy of a third-generation oncolytic herpes simplex virus in neuroendocrine tumor xenograft models.Oncotarget. 2019 Dec 24;10(67):7132-7141. doi: 10.18632/oncotarget.27391. eCollection 2019 Dec 24. Oncotarget. 2019. PMID: 31903171 Free PMC article.
-
Third-generation oncolytic herpes simplex virus inhibits the growth of liver tumors in mice.Cancer Sci. 2018 Mar;109(3):600-610. doi: 10.1111/cas.13492. Epub 2018 Feb 14. Cancer Sci. 2018. PMID: 29288515 Free PMC article.
-
IL-12 Expressing oncolytic herpes simplex virus promotes anti-tumor activity and immunologic control of metastatic ovarian cancer in mice.J Ovarian Res. 2016 Oct 27;9(1):70. doi: 10.1186/s13048-016-0282-3. J Ovarian Res. 2016. PMID: 27784340 Free PMC article.
-
The efficacy of a third-generation oncolytic herpes simplex viral therapy for an HPV-related uterine cervical cancer model.Int J Clin Oncol. 2021 Mar;26(3):591-597. doi: 10.1007/s10147-020-01823-6. Epub 2020 Nov 4. Int J Clin Oncol. 2021. PMID: 33146805
-
Treatment of human hepatocellular carcinoma by the oncolytic herpes simplex virus G47delta.Cancer Cell Int. 2014 Sep 19;14(1):83. doi: 10.1186/s12935-014-0083-y. eCollection 2014. Cancer Cell Int. 2014. PMID: 25360068 Free PMC article.
Cited by
-
New strategies in soft tissue sarcoma treatment.J Hematol Oncol. 2024 Sep 2;17(1):76. doi: 10.1186/s13045-024-01580-3. J Hematol Oncol. 2024. PMID: 39218932 Free PMC article. Review.
-
Advances in Gene Therapy with Oncolytic Viruses and CAR-T Cells and Therapy-Related Groups.Curr Issues Mol Biol. 2025 Apr 10;47(4):268. doi: 10.3390/cimb47040268. Curr Issues Mol Biol. 2025. PMID: 40699667 Free PMC article. Review.
References
-
- Committee on Bone and Soft Tissue Tumors . Japanese Orthopaedic Association/National Cancer Center; 2015. National Bone and Soft Tissue Tumor Registry List 2015.
-
- Fletcher C.D.M., Bridge J.A., Hogendoorn P.C.W. Fourth edition. IARC Press; 2013. World Health Organization Classification of Tumours of Soft Tissue and Bone.
-
- Dry S.M., Fröhling S. In: Soft Tissue and Bone Tumours. Fifth edition. WHO Classification of Tumours Editorial Board, editor. Vol. 3. IARC; 2020. Leiomyosarcoma; pp. 195–197. WHO Classification of Tumours.
-
- Goldblum J.R., Folpe A.L., Weiss S.W., Enzinger F.M. Sixth edition. Mosby/Elsevier; 2014. Enzinger and Weiss’s Soft Tissue Tumors; pp. 549–568.
LinkOut - more resources
Full Text Sources
Research Materials

