Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection
- PMID: 35615280
- PMCID: PMC9124826
- DOI: 10.3389/fnins.2022.883584
Navigated Intraoperative 3D Ultrasound in Glioblastoma Surgery: Analysis of Imaging Features and Impact on Extent of Resection
Abstract
Background: Neuronavigation is routinely used in glioblastoma surgery, but its accuracy decreases during the operative procedure due to brain shift, which can be addressed utilizing intraoperative imaging. Intraoperative ultrasound (iUS) is widely available, offers excellent live imaging, and can be fully integrated into modern navigational systems. Here, we analyze the imaging features of navigated i3D US and its impact on the extent of resection (EOR) in glioblastoma surgery.
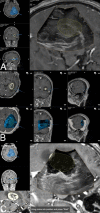
Methods: Datasets of 31 glioblastoma resection procedures were evaluated. Patient registration was established using intraoperative computed tomography (iCT). Pre-operative MRI (pre-MRI) and pre-resectional ultrasound (pre-US) datasets were compared regarding segmented tumor volume, spatial overlap (Dice coefficient), the Euclidean distance of the geometric center of gravity (CoG), and the Hausdorff distance. Post-resectional ultrasound (post-US) and post-operative MRI (post-MRI) tumor volumes were analyzed and categorized into subtotal resection (STR) or gross total resection (GTR) cases.
Results: The mean patient age was 59.3 ± 11.9 years. There was no significant difference in pre-resectional segmented tumor volumes (pre-MRI: 24.2 ± 22.3 cm3; pre-US: 24.0 ± 21.8 cm3). The Dice coefficient was 0.71 ± 0.21, the Euclidean distance of the CoG was 3.9 ± 3.0 mm, and the Hausdorff distance was 12.2 ± 6.9 mm. A total of 18 cases were categorized as GTR, 10 cases were concordantly classified as STR on MRI and ultrasound, and 3 cases had to be excluded from post-resectional analysis. In four cases, i3D US triggered further resection.
Conclusion: Navigated i3D US is reliably adjunct in a multimodal navigational setup for glioblastoma resection. Tumor segmentations revealed similar results in i3D US and MRI, demonstrating the capability of i3D US to delineate tumor boundaries. Additionally, i3D US has a positive influence on the EOR, allows live imaging, and depicts brain shift.
Keywords: brain shift; extent of resection; glioblastoma; intraoperative imaging; intraoperative ultrasound; neuronavigation.
Copyright © 2022 Saß, Zivkovic, Pojskic, Nimsky and Bopp.
Conflict of interest statement
CN and MB are consultants for Brainlab. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Audette M. A., Siddiqi K., Peters T. M. (eds) (1999). Level-Set Surface Segmentation and Fast Cortical Range Image Tracking for Computing Intrasurgical Deformations. Berlin: Springer Berlin Heidelberg.
LinkOut - more resources
Full Text Sources

