Genetic Evidence Reveals the Indispensable Role of the rseC Gene for Autotrophy and the Importance of a Functional Electron Balance for Nitrate Reduction in Clostridium ljungdahlii
- PMID: 35615511
- PMCID: PMC9124969
- DOI: 10.3389/fmicb.2022.887578
Genetic Evidence Reveals the Indispensable Role of the rseC Gene for Autotrophy and the Importance of a Functional Electron Balance for Nitrate Reduction in Clostridium ljungdahlii
Abstract
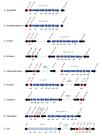
For Clostridium ljungdahlii, the RNF complex plays a key role for energy conversion from gaseous substrates such as hydrogen and carbon dioxide. In a previous study, a disruption of RNF-complex genes led to the loss of autotrophy, while heterotrophy was still possible via glycolysis. Furthermore, it was shown that the energy limitation during autotrophy could be lifted by nitrate supplementation, which resulted in an elevated cellular growth and ATP yield. Here, we used CRISPR-Cas12a to delete: (1) the RNF complex-encoding gene cluster rnfCDGEAB; (2) the putative RNF regulator gene rseC; and (3) a gene cluster that encodes for a putative nitrate reductase. The deletion of either rnfCDGEAB or rseC resulted in a complete loss of autotrophy, which could be restored by plasmid-based complementation of the deleted genes. We observed a transcriptional repression of the RNF-gene cluster in the rseC-deletion strain during autotrophy and investigated the distribution of the rseC gene among acetogenic bacteria. To examine nitrate reduction and its connection to the RNF complex, we compared autotrophic and heterotrophic growth of our three deletion strains with either ammonium or nitrate. The rnfCDGEAB- and rseC-deletion strains failed to reduce nitrate as a metabolic activity in non-growing cultures during autotrophy but not during heterotrophy. In contrast, the nitrate reductase deletion strain was able to grow in all tested conditions but lost the ability to reduce nitrate. Our findings highlight the important role of the rseC gene for autotrophy, and in addition, contribute to understand the connection of nitrate reduction to energy metabolism.
Keywords: CRISPR; Clostridium ljungdahlii; RNF-gene cluster; acetogenic bacteria; autotrophy; nitrate reduction.
Copyright © 2022 Klask, Jäger, Casini, Angenent and Molitor.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
 ,
,  ), and ΔrseC (
), and ΔrseC ( ,
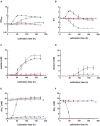
,  ) were grown in 100 mL PETC medium in 1 L bottles at 37°C and 150 rpm. The headspace consisted of H2 and CO2 (80/20 vol-%) and was set to 0.5 bar overpressure. The medium contained either 18.7 mM nitrate (NO3–) (filled circles) or 18.7 mM ammonium (NH4+) (open circles) as nitrogen source. The cultivation times were 173 h for cultures of C. ljungdahlii WT and C. ljungdahlii ΔRNF and 186 h for cultures of C. ljungdahlii ΔrseC. All cultures were grown in biological triplicates, data is given as mean values, with error bars indicating the standard deviation. (A) Growth; (B) pH-behavior; (C) acetate concentrations; (D) ethanol concentration; (E) ammonium concentration; and (F) nitrate concentrations. WT, wild type; ΔRNF, RNF-gene cluster deletion; ΔrseC, rseC gene deletion.
) were grown in 100 mL PETC medium in 1 L bottles at 37°C and 150 rpm. The headspace consisted of H2 and CO2 (80/20 vol-%) and was set to 0.5 bar overpressure. The medium contained either 18.7 mM nitrate (NO3–) (filled circles) or 18.7 mM ammonium (NH4+) (open circles) as nitrogen source. The cultivation times were 173 h for cultures of C. ljungdahlii WT and C. ljungdahlii ΔRNF and 186 h for cultures of C. ljungdahlii ΔrseC. All cultures were grown in biological triplicates, data is given as mean values, with error bars indicating the standard deviation. (A) Growth; (B) pH-behavior; (C) acetate concentrations; (D) ethanol concentration; (E) ammonium concentration; and (F) nitrate concentrations. WT, wild type; ΔRNF, RNF-gene cluster deletion; ΔrseC, rseC gene deletion.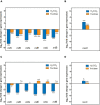
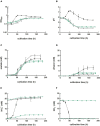
 ) or 18.7 mM ammonium (NH4+) (
) or 18.7 mM ammonium (NH4+) ( ) as nitrogen source. The C. ljungdahlii WT data (●, ◦) from Figure 1 is given for comparison. All cultures were grown in biological triplicates, data is given as mean values, with error bars indicating the standard deviation. (A) Growth; (B) pH-behavior; (C) acetate concentrations; (D) ethanol concentration; (E) ammonium concentration; and (F) nitrate concentrations. Δnar, deletion of nitrate reductase gene cluster; rpm, revolutions per minute; CO2, carbon dioxide; and H2, hydrogen.
) as nitrogen source. The C. ljungdahlii WT data (●, ◦) from Figure 1 is given for comparison. All cultures were grown in biological triplicates, data is given as mean values, with error bars indicating the standard deviation. (A) Growth; (B) pH-behavior; (C) acetate concentrations; (D) ethanol concentration; (E) ammonium concentration; and (F) nitrate concentrations. Δnar, deletion of nitrate reductase gene cluster; rpm, revolutions per minute; CO2, carbon dioxide; and H2, hydrogen.
Similar articles
-
The Rnf complex of Clostridium ljungdahlii is a proton-translocating ferredoxin:NAD+ oxidoreductase essential for autotrophic growth.mBio. 2012 Dec 26;4(1):e00406-12. doi: 10.1128/mBio.00406-12. mBio. 2012. PMID: 23269825 Free PMC article.
-
The Rnf Complex Is an Energy-Coupled Transhydrogenase Essential To Reversibly Link Cellular NADH and Ferredoxin Pools in the Acetogen Acetobacterium woodii.J Bacteriol. 2018 Oct 10;200(21):e00357-18. doi: 10.1128/JB.00357-18. Print 2018 Nov 1. J Bacteriol. 2018. PMID: 30126940 Free PMC article.
-
A genetic system for Clostridium ljungdahlii: a chassis for autotrophic production of biocommodities and a model homoacetogen.Appl Environ Microbiol. 2013 Feb;79(4):1102-9. doi: 10.1128/AEM.02891-12. Epub 2012 Nov 30. Appl Environ Microbiol. 2013. PMID: 23204413 Free PMC article.
-
Hypothesis on the Synchronistic Evolution of Autotrophy and Heterotrophy.Trends Biochem Sci. 2018 Jun;43(6):402-411. doi: 10.1016/j.tibs.2018.03.008. Epub 2018 Apr 11. Trends Biochem Sci. 2018. PMID: 29655512 Review.
-
Engineering Clostridium ljungdahlii as the gas-fermenting cell factory for the production of biofuels and biochemicals.Curr Opin Chem Biol. 2020 Dec;59:54-61. doi: 10.1016/j.cbpa.2020.04.010. Epub 2020 May 29. Curr Opin Chem Biol. 2020. PMID: 32480247 Review.
Cited by
-
Translational efficiency in gas-fermenting bacteria: Adding a new layer of regulation to gene expression in acetogens.iScience. 2023 Nov 2;26(12):108383. doi: 10.1016/j.isci.2023.108383. eCollection 2023 Dec 15. iScience. 2023. PMID: 38034355 Free PMC article. Review.
-
Functional Characterization of RseC in the SoxR Reducing System and Its Role in Oxidative Stress Response in Escherichia coli.J Microbiol Biotechnol. 2024 Dec 28;34(12):2547-2554. doi: 10.4014/jmb.2410.10007. Epub 2024 Nov 25. J Microbiol Biotechnol. 2024. PMID: 39631780 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

