The effect and therapeutic compliance of adjuvant therapy in patients with cholangiocarcinoma after R0 resection: a retrospective study
- PMID: 35615785
- PMCID: PMC9946916
- DOI: 10.12701/jyms.2022.00213
The effect and therapeutic compliance of adjuvant therapy in patients with cholangiocarcinoma after R0 resection: a retrospective study
Abstract
Backgruound: This study aimed to compare clinical outcomes between surveillance and adjuvant therapy (AT) groups after R0 resection for cholangiocarcinoma (CCA).
Methods: A total of 154 patients who underwent R0 resection for CCA at the Daegu Catholic University Medical Center between January 2010 and December 2019 were included. Overall survival (OS) and progression-free survival (PFS) were analyzed.
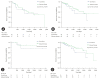
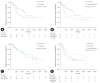
Results: The median follow-up duration was 899 days. There were 109 patients in the AT group and 45 patients in the surveillance group. The patients in the AT group were younger (67 years vs. 74 years, p<0.001) and included more males (64.2% vs. 46.7%, p=0.044). The proportion of patients with stage III CCA was larger in the AT group than in the surveillance group (13.8% vs. 2.2%, p=0.005). In addition, AT did not improve OS (5-year OS rate, 69.3% in the AT group vs. 64.2% in the surveillance group, p=0.806) or PFS (5-year PFS rate, 42.6% in the AT group vs. 48.9% in the surveillance group, p=0.113). In multivariate analysis using the Cox proportional hazards model, stage III CCA (hazard ratio [HR], 10.81; 95% confidence interval [CI], 2.92-40.00; p<0.001) was a significant predictor of OS. American Society of Anesthesiologists classification II (HR, 0.50; 95% CI, 0.31-0.81; p=0.005), and American Joint Committee on Cancer stages II (HR, 3.14; 95% CI, 1.25-7.89; p=0.015) and III (HR, 8.08; 95% CI, 2.80-23.32; p<0.001) were independent predictors of PFS.
Conclusion: AT after R0 resection for CCA did not improve OS or PFS.
Keywords: Adjuvant chemotherapy; Biliary tract surgical procedures; Cholangiocarcinoma; Survival analysis; Watchful waiting.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures




References
-
- Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: epidemiology and risk factors. Liver Int. 2019;39(Suppl 1):19–31. - PubMed
-
- Belkouz A, Wilmink JW, Haj Mohammad N, Hagendoorn J, de Vos-Geelen J, Dejong CH, et al. Advances in adjuvant therapy of biliary tract cancer: an overview of current clinical evidence based on phase II and III trials. Crit Rev Oncol Hematol. 2020;151:102975. - PubMed
-
- Allen MJ, Knox JJ. A review of current adjuvant and neoadjuvant systemic treatments for cholangiocarcinoma and gallbladder carcinoma. Hepatoma Res. 2021;7:73.
LinkOut - more resources
Full Text Sources

