Signaling mechanisms of the platelet glycoprotein Ib-IX complex
- PMID: 35615944
- PMCID: PMC9378482
- DOI: 10.1080/09537104.2022.2071852
Signaling mechanisms of the platelet glycoprotein Ib-IX complex
Abstract
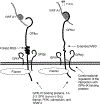
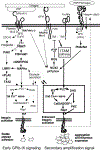
The glycoprotein Ib-IX (GPIb-IX) complex mediates initial platelet adhesion to von Willebrand factor (VWF) immobilized on subendothelial matrix and endothelial surfaces, and transmits VWF binding-induced signals to stimulate platelet activation. GPIb-IX also functions as part of a mechanosensor to convert mechanical force received via VWF binding into intracellular signals, thereby greatly enhancing platelet activation. Thrombin binding to GPIb-IX initiates GPIb-IX signaling cooperatively with protease-activated receptors to synergistically stimulate the platelet response to low-dose thrombin. GPIb-IX signaling may also occur following the binding of other GPIb-IX ligands such as leukocyte integrin αMβ2 and red cell-derived semaphorin 7A, contributing to thrombo-inflammation. GPIb-IX signaling requires the interaction between the cytoplasmic domains of GPIb-IX and 14-3-3 protein and is mediated through Src family kinases, the Rho family of small GTPases, phosphoinositide 3-kinase-Akt-cGMP-mitogen-activated protein kinase, and LIM kinase 1 signaling pathways, leading to calcium mobilization, integrin activation, and granule secretion. This review summarizes the current understanding of GPIb-IX signaling.
Keywords: Glycoprotein Ib (GPIb); integrin; intracellular signaling; platelet activation; thrombosis; von Willebrand factor.
Figures
References
-
- Ware J Molecular analyses of the platelet glycoprotein Ib-IX-V receptor. Thromb Haemost 1998;79:466–478. - PubMed
-
- Caen JP, Nurden AT, Jeanneau C, Michel H, Tobelem G, Levy-Toledano S, Sultan Y, Valensi F, Bernard J. Bernard-Soulier syndrome: a new platelet glycoprotein abnormality. Its relationship with platelet adhesion to subendothelium and with the factor VIII von Willebrand protein. J Lab Clin Med 1976;87:586–596. Epub 1976/04/01. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
