Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signaling to Promote Health and Longevity
- PMID: 35616058
- PMCID: PMC9203038
- DOI: 10.1161/CIRCULATIONAHA.122.059863
Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signaling to Promote Health and Longevity
Abstract
Background: The insulin-like growth factor 1 (IGF1) pathway is a key regulator of cellular metabolism and aging. Although its inhibition promotes longevity across species, the effect of attenuated IGF1 signaling on cardiac aging remains controversial.
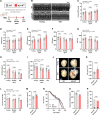
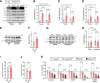
Methods: We performed a lifelong study to assess cardiac health and lifespan in 2 cardiomyocyte-specific transgenic mouse models with enhanced versus reduced IGF1 receptor (IGF1R) signaling. Male mice with human IGF1R overexpression or dominant negative phosphoinositide 3-kinase mutation were examined at different life stages by echocardiography, invasive hemodynamics, and treadmill coupled to indirect calorimetry. In vitro assays included cardiac histology, mitochondrial respiration, ATP synthesis, autophagic flux, and targeted metabolome profiling, and immunoblots of key IGF1R downstream targets in mouse and human explanted failing and nonfailing hearts, as well.
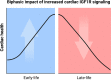
Results: Young mice with increased IGF1R signaling exhibited superior cardiac function that progressively declined with aging in an accelerated fashion compared with wild-type animals, resulting in heart failure and a reduced lifespan. In contrast, mice with low cardiac IGF1R signaling exhibited inferior cardiac function early in life, but superior cardiac performance during aging, and increased maximum lifespan, as well. Mechanistically, the late-life detrimental effects of IGF1R activation correlated with suppressed autophagic flux and impaired oxidative phosphorylation in the heart. Low IGF1R activity consistently improved myocardial bioenergetics and function of the aging heart in an autophagy-dependent manner. In humans, failing hearts, but not those with compensated hypertrophy, displayed exaggerated IGF1R expression and signaling activity.
Conclusions: Our findings indicate that the relationship between IGF1R signaling and cardiac health is not linear, but rather biphasic. Hence, pharmacological inhibitors of the IGF1 pathway, albeit unsuitable for young individuals, might be worth considering in older adults.
Keywords: aging; autophagy; cardiomyopathies; insulin-like growth factor 1; mitochondria; mouse; phosphatidylinositol 3-kinases.
Figures



Comment in
-
Spermidine overrides INSR (insulin receptor)-IGF1R (insulin-like growth factor 1 receptor)-mediated inhibition of autophagy in the aging heart.Autophagy. 2022 Oct;18(10):2500-2502. doi: 10.1080/15548627.2022.2095835. Epub 2022 Jul 10. Autophagy. 2022. PMID: 35786404 Free PMC article.
-
Cardiac PI3K p110α attenuation delays aging and extends lifespan.Cell Stress. 2022 Aug 8;6(8):72-75. doi: 10.15698/cst2022.08.270. eCollection 2022 Aug. Cell Stress. 2022. PMID: 36447531 Free PMC article.
-
Response by Sedej and Abdellatif to Letter Regarding Article, "Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signaling to Promote Health and Longevity".Circulation. 2022 Dec 13;146(24):e332-e333. doi: 10.1161/CIRCULATIONAHA.122.062496. Epub 2022 Dec 12. Circulation. 2022. PMID: 36508494 No abstract available.
-
Letter by Xie and Hou Regarding Article, "Fine-Tuning Cardiac Insulin-Like Growth Factor 1 Receptor Signaling to Promote Health and Longevity".Circulation. 2022 Dec 13;146(24):e331. doi: 10.1161/CIRCULATIONAHA.122.061531. Epub 2022 Dec 12. Circulation. 2022. PMID: 36508496 No abstract available.
References
-
- Johnson SC. Nutrient sensing, signaling and ageing: the role of IGF-1 and mTOR in ageing and age-related disease. Subcell Biochem. 2018;90:49–97. doi: 10.1007/978-981-13-2835-0_3 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

