Activation of the Lateral Habenula-Ventral Tegmental Area Neural Circuit Contributes to Postoperative Cognitive Dysfunction in Mice
- PMID: 35616407
- PMCID: PMC9353455
- DOI: 10.1002/advs.202202228
Activation of the Lateral Habenula-Ventral Tegmental Area Neural Circuit Contributes to Postoperative Cognitive Dysfunction in Mice
Abstract
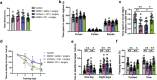
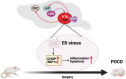
Postoperative cognitive dysfunction (POCD) is common and is associated with poor outcome. Neural circuit involvement in POCD is unknown. Lateral habenula (LHb) that regulates coping and depression-like behaviors after aversive stimuli is activated by surgery in the previous study. Here, surgery activated LHb and ventral tegmental area (VTA) are presented. VTA is known to receive projections from LHb and project to the prefrontal cortex and hippocampus. Direct chemogenetic inhibition of LHb or damaging LHb attenuates surgery-induced learning and memory impairment, N-methyl-d-aspartate (NMDA) receptor activation, endoplasmic reticulum stress, inflammatory responses and cell injury in the VTA, and activation of rostromedial tegmental nucleus, an intermediate station to connect LHb with VTA. LHb inhibition preserves dendritic spine density in the prefrontal cortex and hippocampus. Retrograde inhibition of LHb via its projections to VTA attenuated surgery-induced learning and memory dysfunction is observed. Retrograde activation of LHb induced learning and memory dysfunction is observed. Inhibition of NMDA receptors, dopamine synthesis, and endoplasmic reticulum stress in the VTA reduced surgery-induced learning and memory impairment, inflammatory responses, and cell injury are observed. These results suggest that surgery activates the LHb-VTA neural circuit, which contributes to POCD and neuropathological changes in the brain. These novel findings represent initial evidence for neural circuit involvement in surgery effects.
Keywords: chemogenetic inhibition; endoplasmic reticulum stress; lateral habenula; postoperative cognitive dysfunction; ventral tegmental area.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Monk T. G., Weldon B. C., Garvan C. W., Dede D. E., van der Aa M. T., Heilman K. M., Gravenstein J. S., Anesthesiology 2008, 108, 18. - PubMed
-
- Berger M., Schenning K. J., Brown C. H., Deiner S. G., Whittington R. A., Eckenhoff R. G., Angst M. S., Avramescu S., Bekker A., Brzezinski M., Crosby G., Culley D. J., Eckenhoff M., Eriksson L. I., Evered L., Ibinson J., Kline R. P., Kofke A., Ma D., Mathew J. P., Maze M., Orser B. A., Price C. C., Scott D. A., Silbert B., Su D., Terrando N., Wang D. S., Wei H., Xie Z., Anesth. Analg. 2018, 127, 1406. - PMC - PubMed
-
- Steinmetz J., Christensen K. B., Lund T., Lohse N., Rasmussen L. S., Anesthesiology 2009, 110, 548. - PubMed
-
- Hovens I. B., Schoemaker R. G., van der Zee E. A., Heineman E., Izaks G. J., van Leeuwen B. L., Brain, Behav., Immun. 2012, 26, 1169. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
