Characterization of Elusive Reaction Intermediates Using Infrared Ion Spectroscopy: Application to the Experimental Characterization of Glycosyl Cations
- PMID: 35616920
- PMCID: PMC9219114
- DOI: 10.1021/acs.accounts.2c00040
Characterization of Elusive Reaction Intermediates Using Infrared Ion Spectroscopy: Application to the Experimental Characterization of Glycosyl Cations
Abstract
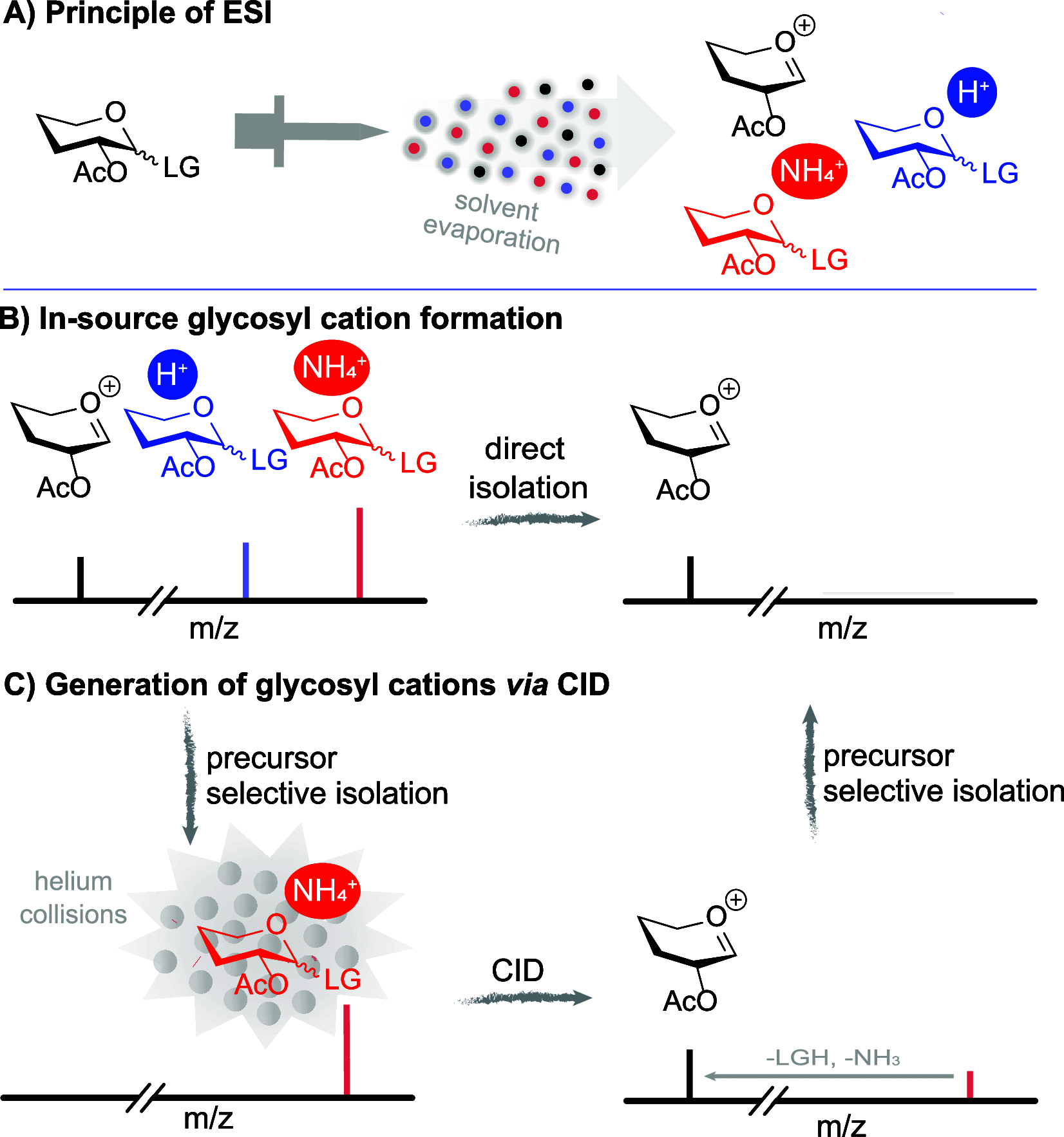
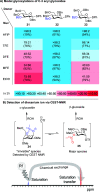
A detailed understanding of the reaction mechanism(s) leading to stereoselective product formation is crucial to understanding and predicting product formation and driving the development of new synthetic methodology. One way to improve our understanding of reaction mechanisms is to characterize the reaction intermediates involved in product formation. Because these intermediates are reactive, they are often unstable and therefore difficult to characterize using experimental techniques. For example, glycosylation reactions are critical steps in the chemical synthesis of oligosaccharides and need to be stereoselective to provide the desired α- or β-diastereomer. It remains challenging to predict and control the stereochemical outcome of glycosylation reactions, and their reaction mechanisms remain a hotly debated topic. In most cases, glycosylation reactions take place via reaction mechanisms in the continuum between SN1- and SN2-like pathways. SN2-like pathways proceeding via the displacement of a contact ion pair are relatively well understood because the reaction intermediates involved can be characterized by low-temperature NMR spectroscopy. In contrast, the SN1-like pathways proceeding via the solvent-separated ion pair, also known as the glycosyl cation, are poorly understood. SN1-like pathways are more challenging to investigate because the glycosyl cation intermediates involved are highly reactive. The highly reactive nature of glycosyl cations complicates their characterization because they have a short lifetime and rapidly equilibrate with the corresponding contact ion pair. To overcome this hurdle and enable the study of glycosyl cation stability and structure, they can be generated in a mass spectrometer in the absence of a solvent and counterion in the gas phase. The ease of formation, stability, and fragmentation of glycosyl cations have been studied using mass spectrometry (MS). However, MS alone provides little information about the structure of glycosyl cations. By combining mass spectrometry (MS) with infrared ion spectroscopy (IRIS), the determination of the gas-phase structures of glycosyl cations has been achieved. IRIS enables the recording of gas-phase infrared spectra of glycosyl cations, which can be assigned by matching to reference spectra predicted from quantum chemically calculated vibrational spectra. Here, we review the experimental setups that enable IRIS of glycosyl cations and discuss the various glycosyl cations that have been characterized to date. The structure of glycosyl cations depends on the relative configuration and structure of the monosaccharide substituents, which can influence the structure through both steric and electronic effects. The scope and relevance of gas-phase glycosyl cation structures in relation to their corresponding condensed-phase structures are also discussed. We expect that the workflow reviewed here to study glycosyl cation structure and reactivity can be extended to many other reaction types involving difficult-to-characterize ionic intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Glycosyl Oxocarbenium Ions: Structure, Conformation, Reactivity, and Interactions.Acc Chem Res. 2021 Jun 1;54(11):2552-2564. doi: 10.1021/acs.accounts.1c00021. Epub 2021 Apr 30. Acc Chem Res. 2021. PMID: 33930267 Free PMC article. Review.
-
Direct Experimental Characterization of Glycosyl Cations by Infrared Ion Spectroscopy.J Am Chem Soc. 2018 May 16;140(19):6034-6038. doi: 10.1021/jacs.8b01236. Epub 2018 May 7. J Am Chem Soc. 2018. PMID: 29656643 Free PMC article.
-
Elucidating reactive sugar-intermediates by mass spectrometry.Commun Chem. 2025 Mar 7;8(1):67. doi: 10.1038/s42004-025-01467-5. Commun Chem. 2025. PMID: 40055429 Free PMC article. Review.
-
Defining the SN1 Side of Glycosylation Reactions: Stereoselectivity of Glycopyranosyl Cations.ACS Cent Sci. 2019 May 22;5(5):781-788. doi: 10.1021/acscentsci.9b00042. Epub 2019 Apr 18. ACS Cent Sci. 2019. PMID: 31139714 Free PMC article.
-
Mechanistic insight into benzylidene-directed glycosylation reactions using cryogenic infrared spectroscopy.Nat Synth. 2024;3(11):1377-1384. doi: 10.1038/s44160-024-00619-0. Epub 2024 Jul 26. Nat Synth. 2024. PMID: 39524531 Free PMC article.
Cited by
-
Real-time capture of reactive intermediates in an enzymatic reaction: insights into a P450-catalyzed oxidation.Chem Sci. 2025 May 19;16(25):11322-11330. doi: 10.1039/d5sc02240a. eCollection 2025 Jun 25. Chem Sci. 2025. PMID: 40438169 Free PMC article.
-
Characterization of elusive rhamnosyl dioxanium ions and their application in complex oligosaccharide synthesis.Nat Commun. 2024 Mar 13;15(1):2257. doi: 10.1038/s41467-024-46522-2. Nat Commun. 2024. PMID: 38480691 Free PMC article.
-
Stabilization of Glucosyl Dioxolenium Ions by "Dual Participation" of the 2,2-Dimethyl-2-(ortho-nitrophenyl)acetyl (DMNPA) Protection Group for 1,2-cis-Glucosylation.J Org Chem. 2022 Jul 15;87(14):9139-9147. doi: 10.1021/acs.joc.2c00808. Epub 2022 Jun 24. J Org Chem. 2022. PMID: 35748115 Free PMC article.
-
Mechanism of C-3 Acyl Neighboring Group Participation in Mannuronic Acid Glycosyl Donors.J Am Chem Soc. 2025 Jan 8;147(1):932-944. doi: 10.1021/jacs.4c13910. Epub 2024 Dec 18. J Am Chem Soc. 2025. PMID: 39692559 Free PMC article.
-
Competing C-4 and C-5-Acyl Stabilization of Uronic Acid Glycosyl Cations.Chemistry. 2022 Nov 11;28(63):e202201724. doi: 10.1002/chem.202201724. Epub 2022 Sep 12. Chemistry. 2022. PMID: 35959853 Free PMC article.
References
-
- Hansen T.; Elferink H.; van Hengst J. M. A.; Houthuijs K. J.; Remmerswaal W. A.; Kromm A.; Berden G.; van der Vorm S.; Rijs A. M.; Overkleeft H. S.; Filippov D. V.; Rutjes F. P. J. T.; van der Marel G. A.; Martens J.; Oomens J.; Codee J. D. C.; Boltje T. J. Characterization of glycosyl dioxolenium ions and their role in glycosylation reactions. Nat. Commun. 2020, 11, 2664.10.1038/s41467-020-16362-x. - DOI - PMC - PubMed
-
- Szymanski C.; Schnaar R.; Aebi M.; Varki E. A. In Essentials of Glycobiology, 3rd ed.; Varki A., Cummings R. D., Esko J. D., Stanley P., Hart G. W., Aebi M., Darvill A. G., Kinoshita T., Packer N. H., Prestegard J. H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, 2017; Bacterial and Viral Infections.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

