Disability Progression in Multiple Sclerosis Patients using Early First-line Treatments
- PMID: 35617144
- PMCID: PMC9544933
- DOI: 10.1111/ene.15422
Disability Progression in Multiple Sclerosis Patients using Early First-line Treatments
Abstract
Background: Therapeutic management of relapsing-remitting multiple sclerosis (RRMS) has evolved towards early treatment. The objective was to assess the impact of early treatment initiation on disability progression among RRMS first-line treated patients.
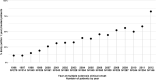
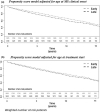
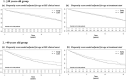
Methods: This study included all incident RRMS cases starting interferon or glatiramer acetate for the first time from 1996/01/01 to 2012/31/12 (N=5,279) from ten MS expert OFSEP centers (Observatoire Français de la Sclérose en Plaques). The delay from treatment start to attain an irreversible Expanded Disability Status Scale score of 3.0 were compared between "Early" group (N= 1,882; treated within 12 months following MS clinical onset) and "Later" group using propensity score weighted Kaplan-Meier methods, overall and stratified by age.
Results: Overall, the restricted mean time before reaching EDSS 3.0 (RMST) from treatment start was 11 years and two months for patients treated within the year following MS clinical onset and 10 years and seven months for patients treated later. Thus, early treated patients gained 7 months (95% CI: [4-11] months) in the time to reach EDSS 3.0 compared to patients treated later (treatment start delayed by 28 months). The difference in RMST was respectively six months (95% CI: [1-10] months) and 14 months (95% CI: [4-24] months) in the "≤40 years" age group and in the ">40 years" age group, in favour of early group. .
Conclusions: Early treatment initiation resulted in a significant reduction of disability progression among patients with RRMS, and also among older patients.
Keywords: beta-interferon; disability progression; early treatment; glatiramer acetate; multiple sclerosis; observational studies; propensity score.
This article is protected by copyright. All rights reserved.
Conflict of interest statement
M. Lefort reports that she had travel grants from Roche SAS. S. Vukusic reports that she had consulting and lecturing fees, travel grants and unconditional research support from Biogen, Celgène, Geneuro, Genzyme, MedDay, Merck Serono, Novartis, Roche, Sanofi Aventis and Teva Pharma. R. Casey reports that he had no conflict of interest. G. Edan reports that he had personal honoraria for lectures or consulting from Bayer, Biogen, LFB, Merck, Novartis, Roche, Sanofi; research support from Bayer, Biogen, Genzyme, Merck, Novartis, Roche and Teva Pharma. E. Leray reports that she had consulting and lecture fees or travel grants from Biogen, Genzyme, MedDay Pharmaceuticals, Merck Serono, Novartis, Sanofi Aventis and Roche.
Figures


References
-
- Montalban X, Gold R, Thompson AJ, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. 2018;24(2):96‐120. - PubMed
-
- Tintoré M. New options for early treatment of multiple sclerosis. J Neurol Sci. 2009;277(SUPPL. 1):9‐11. - PubMed
-
- Kappos L, Freedman MS, Polman CH, et al. Long‐term effect of early treatment with interferon beta‐1b after a first clinical event suggestive of multiple sclerosis: 5‐year active treatment extension of the phase 3 BENEFIT trial. Lancet Neurol. 2009;8(11):987‐997. doi:10.1016/S1474-4422(09)70237-6 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

