Mobilization-based chemotherapy-free engraftment of gene-edited human hematopoietic stem cells
- PMID: 35617958
- PMCID: PMC9240327
- DOI: 10.1016/j.cell.2022.04.039
Mobilization-based chemotherapy-free engraftment of gene-edited human hematopoietic stem cells
Abstract
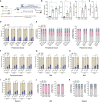
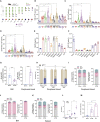
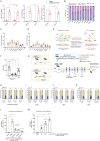
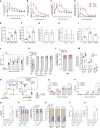
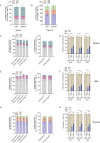
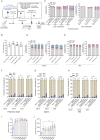
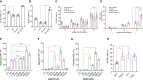
Hematopoietic stem/progenitor cell gene therapy (HSPC-GT) is proving successful to treat several genetic diseases. HSPCs are mobilized, harvested, genetically corrected ex vivo, and infused, after the administration of toxic myeloablative conditioning to deplete the bone marrow (BM) for the modified cells. We show that mobilizers create an opportunity for seamless engraftment of exogenous cells, which effectively outcompete those mobilized, to repopulate the depleted BM. The competitive advantage results from the rescue during ex vivo culture of a detrimental impact of mobilization on HSPCs and can be further enhanced by the transient overexpression of engraftment effectors exploiting optimized mRNA-based delivery. We show the therapeutic efficacy in a mouse model of hyper IgM syndrome and further developed it in human hematochimeric mice, showing its applicability and versatility when coupled with gene transfer and editing strategies. Overall, our findings provide a potentially valuable strategy paving the way to broader and safer use of HSPC-GT.
Keywords: CRISPR-Cas gene editing; RNA-based delivery; X-linked hyper-IgM syndrome; autologous stem cell transplantation; conditioning-free; gene transfer; hematopoietic stem cells; human hematochimeric mouse model; mobilization.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests L.N. is the inventor of patents on the applications of gene editing in HSPCs, owned and managed by the San Raffaele Scientific Institute and the Telethon Foundation, including the improved gene editing filed by L.N., S.F., A.J., and M.F., the increasing engraftment by HSPCs filed by L.N., C.L. and M.M. and the mobilization-based HSCT filed by L.N. and A.O.-J. L.N. is a co-founder and quota holder of GeneSpire, a startup company aiming to develop ex vivo gene editing in genetic diseases.
Figures





References
-
- Andreani M., Testi M., Gaziev J., Condello R., Bontadini A., Tazzari P.L., Ricci F., De Felice L.D., Agostini F., Fraboni D., et al. Quantitatively different red cell/nucleated cell chimerism in patients with long-term, persistent hematopoietic mixed chimerism after bone marrow transplantation for thalassemia major or sickle cell disease. Haematologica. 2011;96:128–133. - PMC - PubMed
-
- Balashov D., Laberko A., Shcherbina A., Trakhtman P., Abramov D., Gutovskaya E., Kozlovskaya S., Shelikhova L., Novichkova G., Maschan M., et al. A conditioning regimen with plerixafor is safe and improves the outcome of TCRαβ+ and CD19+ cell-depleted stem cell transplantation in patients with Wiskott-Aldrich syndrome. Biol. Blood Marrow Transplant. 2018;24:1432–1440. - PubMed
-
- Bendall L.J., Bradstock K.F. G-CSF: from granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014;25:355–367. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

