Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma
- PMID: 35618286
- PMCID: PMC9125767
- DOI: 10.1136/jitc-2021-004297
Bufalin stimulates antitumor immune response by driving tumor-infiltrating macrophage toward M1 phenotype in hepatocellular carcinoma
Abstract
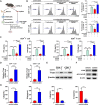
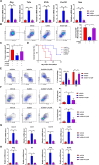
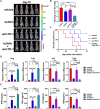
Background: Immunotherapy for hepatocellular carcinoma (HCC) exhibits limited clinical efficacy due to immunosuppressive tumor microenvironment (TME). Tumor-infiltrating macrophages (TIMs) account for the major component in the TME, and the dominance of M2 phenotype over M1 phenotype in the TIMs plays the pivotal role in sustaining the immunosuppressive character. We thus investigate the effect of bufalin on promoting TIMs polarization toward M1 phenotype to improve HCC immunotherapy.
Methods: The impact of bufalin on evoking antitumor immune response was evaluated in the immunocompetent mouse HCC model. The expression profiling of macrophage-associated genes, surface markers and cytokines on bufalin treatment in vitro and in vivo were detected using flow cytometry, immunofluorescence, western blot analysis, ELISA and RT-qPCR. Cell signaling involved in M1 macrophage polarization was identified via the analysis of gene sequencing, and bufalin-governed target was explored by immunoprecipitation, western blot analysis and gain-and-loss of antitumor immune response. The combination of bufalin and antiprogrammed cell death protein 1 (anti-PD-1) antibody was also assessed in orthotopic HCC mouse model.
Results: In this study, we showed that bufalin can function as an antitumor immune modulator that governs the polarization of TIMs from tumor-promoting M2 toward tumor-inhibitory M1, which induces HCC suppression through the activation of effector T cell immune response. Mechanistically, bufalin inhibits overexpression of p50 nuclear factor kappa B (NF-κB) factor, leading to the predominance of p65-p50 heterodimers over p50 homodimers in the nuclei. The accumulation of p65-p50 heterodimers activates NF-κB signaling, which is responsible for the production of immunostimulatory cytokines, thus resulting in the activation of antitumor T cell immune response. Moreover, bufalin enhances the antitumor activity of anti-PD-1 antibody, and the combination exerts synergistic effect on HCC suppression.
Conclusions: These data expound a novel antitumor mechanism of bufalin, and facilitate exploitation of a new potential macrophage-based HCC immunotherapeutic modality.
Keywords: drug therapy, combination; immunotherapy; liver neoplasms; macrophages; tumor microenvironment.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
