Real-life experience of ledipasvir and sofosbuvir for HCV infected Korean patients: a multicenter cohort study
- PMID: 35618302
- PMCID: PMC9666263
- DOI: 10.3904/kjim.2022.013
Real-life experience of ledipasvir and sofosbuvir for HCV infected Korean patients: a multicenter cohort study
Abstract
Background/aims: To evaluate the efficacy and safety of ledipasvir/sofosbuvir (LDV/SOF) therapy in hepatitis C virus (HCV)-infected Korean patients in a real clinical setting.
Methods: A total of 273 patients who received LDV/SOF therapy between May 2016 and February 2021 were consecutively enrolled and analyzed. A per-protocol analysis was performed to evaluate the virologic response.
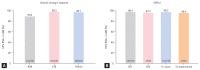
Results: Seventy-five percent were infected with genotype 1, and 25% were infected with genotype 2. A hundred eightyone (66.3%) patients had chronic hepatitis, 74 (27.1%) had compensated cirrhosis, eight (2.9%) had decompensated cirrhosis, and 10 (3.7%) had undergone liver transplantation. Undetectable HCV RNA at week 4 was achieved in 90.2% (231/256) of patients, 99.2% (250/252) achieved the end of treatment response, and 98.1% (202/206) achieved sustained virologic response at 12 weeks post-treatment (SVR12). According to liver function, the SVR12 rates were 99.3% (135/136) in chronic hepatitis, 96.4% (53/55) in compensated cirrhosis, and 100% (6/6) in decompensated cirrhosis. The SVR12 rates according to the genotype were 98.2% (167/170) for genotype 1 and 97.2% (35/36) for genotype 2. An 8-week LDV/SOF treatment in treatment-naïve chronic hepatitis patients with HCV RNA < 6,000,000 IU/mL at baseline resulted in 100% (23/23) SVR12 rates. Overall, LDV/SOF was tolerated well, with a 0.7% (2/273) discontinuation rate due to adverse events that were unrelated to LDV/SOF.
Conclusion: LDV/SOF is effective and safe for treating HCV-infected Korean patients with high SVR12 rates.
Keywords: Genotype; Hepatitis C virus; Ledipasvir; Sofosbuvir; Sustained virologic response.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
Effectiveness and safety of sofosbuvir-based regimens plus an NS5A inhibitor for patients with HCV genotype 3 infection and cirrhosis. Results of a multicenter real-life cohort.J Viral Hepat. 2017 Apr;24(4):304-311. doi: 10.1111/jvh.12648. Epub 2016 Dec 9. J Viral Hepat. 2017. PMID: 27935168
-
Real life efficacy of ledipasvir/sofosbuvir in hepatitis C genotype 4-infected patients with advanced liver fibrosis and decompensated cirrhosis.J Infect. 2018 Jun;76(6):536-542. doi: 10.1016/j.jinf.2018.04.001. Epub 2018 May 6. J Infect. 2018. PMID: 29742470
-
Safety and Effectiveness of Ledipasvir and Sofosbuvir, With or Without Ribavirin, in Treatment-Experienced Patients With Genotype 1 Hepatitis C Virus Infection and Cirrhosis.Clin Gastroenterol Hepatol. 2018 Nov;16(11):1811-1819.e4. doi: 10.1016/j.cgh.2017.12.037. Epub 2018 Jan 3. Clin Gastroenterol Hepatol. 2018. PMID: 29306043 Free PMC article.
-
Fixed-dose combination of sofosbuvir and ledipasvir for the treatment of chronic hepatitis C genotype 1.Expert Opin Pharmacother. 2015 Apr;16(5):739-48. doi: 10.1517/14656566.2015.1013938. Epub 2015 Feb 13. Expert Opin Pharmacother. 2015. PMID: 25676581 Review.
-
Ledipasvir-Sofosbuvir for Treating Chronic Hepatitis C: A NICE Single Technology Appraisal-An Evidence Review Group Perspective.Pharmacoeconomics. 2016 Aug;34(8):741-50. doi: 10.1007/s40273-016-0387-y. Pharmacoeconomics. 2016. PMID: 26892974 Review.
Cited by
-
Prognosis Following Sustained Virologic Response in Korean Chronic Hepatitis C Patients Treated with Sofosbuvir-Based Treatment: Data from a Multicenter Prospective Observational Study up to 7 Years.Medicina (Kaunas). 2024 Jul 14;60(7):1132. doi: 10.3390/medicina60071132. Medicina (Kaunas). 2024. PMID: 39064561 Free PMC article.
References
-
- Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. - PubMed
-
- Polaris Observatory HCV Collaborators Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol. 2017;2:161–176. - PubMed
-
- European Association for the Study of the Liver; European Association for the Study of the Liver EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69:461–511. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
