Efficacy of bivalent vaccines of porcine circovirus type 2 and Mycoplasma hyopneumoniae in specific pathogen-free pigs challenged with porcine circovirus type 2d
- PMID: 35618321
- PMCID: PMC9149493
- DOI: 10.4142/jvs.21287
Efficacy of bivalent vaccines of porcine circovirus type 2 and Mycoplasma hyopneumoniae in specific pathogen-free pigs challenged with porcine circovirus type 2d
Abstract
Background: Porcine circovirus type 2 (PCV2) and Mycoplasma hyopneumoniae (MHP) are economically significant pathogens in the pig industry. The use of combined vaccines against PCV2 and MHP is one of the most effective ways of protecting pigs from both diseases, and it has become a part of general management.
Objectives: This study evaluated the efficacy of two new bivalent vaccines of PCV2 and MHP (Myco-X and Myco-XD) in SPF pigs. Myco-X and Myco-XD are a combined vaccine of MHP with PCV2b and PCV2d, respectively.
Methods: Sixteen pigs were divided into four groups: Myco-X-vaccinated challenged, Myco-XD-vaccinated challenged, unvaccinated challenged, and unvaccinated unchallenged. Two milliliters of Myco-X were administered intramuscularly, and 0.5 mL of Myco-XD was injected intradermally at 3 wk of age. The pigs were challenged with virulent PCV2d via the intramuscular and intranasal route 4 wk post-vaccination.
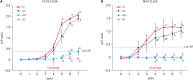
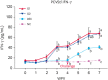
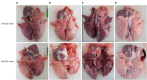
Results: All vaccinated pigs showed effective reduction of the clinical signs, the PCV2d load in the blood and nasal swab samples, as well as lung and lymphoid tissue lesions in the challenge test. Compared to unvaccinated challenged animals, the vaccinated challenged animals showed significantly higher (p < 0.05) levels of anti-PCV2 IgG, PCV2d-specific interferon-γ (IFN-γ), and anti-MHP IgG.
Conclusions: Based on clinical, microbiological, serological, and pathological assessments, this study confirmed that both combined vaccines could protect pigs against PCV2 infection caused by PCV2d. On the other hand, further research on the efficacy evaluation of these new vaccines against the MHP challenge and PCV2d/MHP co-challenge is needed.
Keywords: Mycoplasma hyopneumoniae; Porcine circovirus; bivalent vaccine.
© 2022 The Korean Society of Veterinary Science.
Conflict of interest statement
This experiment was carried out independently without any directions from the vaccine company. The authors declared no conflicts of interest.
Figures




References
-
- Chae C. A review of porcine circovirus 2-associated syndromes and diseases. Vet J. 2005;169(3):326–336. - PubMed
-
- Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591–615. - PubMed
-
- An DJ, Roh IS, Song DS, Park CK, Park BK. Phylogenetic characterization of porcine circovirus type 2 in PMWS and PDNS Korean pigs between 1999 and 2006. Virus Res. 2007;129(2):115–122. - PubMed
-
- Kwon T, Lee DU, Yoo SJ, Je SH, Shin JY, Lyoo YS. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Res. 2017;228:24–29. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

