Application of 7T MRS to High-Grade Gliomas
- PMID: 35618424
- PMCID: PMC9575545
- DOI: 10.3174/ajnr.A7502
Application of 7T MRS to High-Grade Gliomas
Abstract
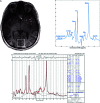
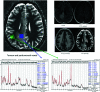
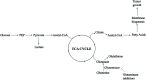
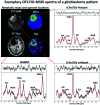
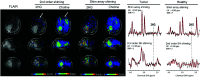
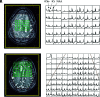
MRS, including single-voxel spectroscopy and MR spectroscopic imaging, captures metabolites in high-grade gliomas. Emerging evidence indicates that 7T MRS may be more sensitive to aberrant metabolic activity than lower-field strength MRS. However, the literature on the use of 7T MRS to visualize high-grade gliomas has not been summarized. We aimed to identify metabolic information provided by 7T MRS, optimal spectroscopic sequences, and areas for improvement in and new applications for 7T MRS. Literature was found on PubMed using "high-grade glioma," "malignant glioma," "glioblastoma," "anaplastic astrocytoma," "7T," "MR spectroscopy," and "MR spectroscopic imaging." 7T MRS offers higher SNR, modestly improved spatial resolution, and better resolution of overlapping resonances. 7T MRS also yields reduced Cramér-Rao lower bound values. These features help to quantify D-2-hydroxyglutarate in isocitrate dehydrogenase 1 and 2 gliomas and to isolate variable glutamate, increased glutamine, and increased glycine with higher sensitivity and specificity. 7T MRS may better characterize tumor infiltration and treatment effect in high-grade gliomas, though further study is necessary. 7T MRS will benefit from increased sample size; reductions in field inhomogeneity, specific absorption rate, and acquisition time; and advanced editing techniques. These findings suggest that 7T MRS may advance understanding of high-grade glioma metabolism, with reduced Cramér-Rao lower bound values and better measurement of smaller metabolite signals. Nevertheless, 7T is not widely used clinically, and technical improvements are necessary. 7T MRS isolates metabolites that may be valuable therapeutic targets in high-grade gliomas, potentially resulting in wider ranging neuro-oncologic applications.
© 2022 by American Journal of Neuroradiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
