Gold Nanostrip Array-Mediated Wireless Electrical Stimulation for Accelerating Functional Neuronal Differentiation
- PMID: 35618610
- PMCID: PMC9353484
- DOI: 10.1002/advs.202202376
Gold Nanostrip Array-Mediated Wireless Electrical Stimulation for Accelerating Functional Neuronal Differentiation
Abstract
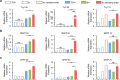
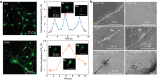
Neural stem cell (NSC)-based therapy holds great promise for the treatment of neurodegenerative diseases. Presently, however, it is hindered by poor functional neuronal differentiation. Electrical stimulation is considered one of the most effective ways to promote neuronal differentiation of NSCs. In addition to surgically implanted electrodes, traditional electrical stimulation includes wires connected to the external power supply, and an additional surgery is required to remove the electrodes or wires following stimulation, which may cause secondary injuries and infections. Herein, a novel method is reported for generation of wireless electrical signals on an Au nanostrip array by leveraging the effect of electromagnetic induction under a rotating magnetic field. The intensity of the generated electrical signals depends on the rotation speed and magnetic field strength. The Au nanostrip array-mediated electric stimulation promotes NSC differentiation into mature neurons within 5 days, at the mRNA, protein, and function levels. The rate of differentiation is faster by at least 5 days than that in cells without treatment. The Au nanostrip array-based wireless device also accelerates neuronal differentiation of NSCs in vivo. The novel method to accelerate the neuronal differentiation of NSCs has the advantages of wireless, timely, localized and precise controllability, and noninvasive power supplementation.
Keywords: NSC-based therapy; electromagnetic induction; mature functional neuron; neuronal differentiation; wireless electrical stimulation.
© 2022 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Electromagnetic Cellularized Patch with Wirelessly Electrical Stimulation for Promoting Neuronal Differentiation and Spinal Cord Injury Repair.Adv Sci (Weinh). 2024 Aug;11(30):e2307527. doi: 10.1002/advs.202307527. Epub 2024 Jun 13. Adv Sci (Weinh). 2024. PMID: 38868910 Free PMC article.
-
Nanoelectrode-Mediated Extracellular Electrical Stimulation Directing Dopaminergic Neuronal Differentiation of Stem Cells for Improved Parkinson's Disease Therapy.Adv Mater. 2025 Feb;37(6):e2409745. doi: 10.1002/adma.202409745. Epub 2024 Dec 20. Adv Mater. 2025. PMID: 39703114
-
Strategy for Designing a Cell Scaffold to Enable Wireless Electrical Stimulation for Enhanced Neuronal Differentiation of Stem Cells.Adv Healthc Mater. 2021 Jun;10(11):e2100027. doi: 10.1002/adhm.202100027. Epub 2021 Apr 22. Adv Healthc Mater. 2021. PMID: 33887103
-
Electrical stimulation affects neural stem cell fate and function in vitro.Exp Neurol. 2019 Sep;319:112963. doi: 10.1016/j.expneurol.2019.112963. Epub 2019 May 21. Exp Neurol. 2019. PMID: 31125549 Review.
-
Electric field-induced effects on neuronal cell biology accompanying dielectrophoretic trapping.Adv Anat Embryol Cell Biol. 2003;173:III-IX, 1-77. doi: 10.1007/978-3-642-55469-8. Adv Anat Embryol Cell Biol. 2003. PMID: 12901336 Review.
Cited by
-
Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury.Nat Commun. 2024 Nov 6;15(1):9580. doi: 10.1038/s41467-024-53858-2. Nat Commun. 2024. PMID: 39505863 Free PMC article.
-
Advances in electroactive biomaterials: Through the lens of electrical stimulation promoting bone regeneration strategy.J Orthop Translat. 2024 Jun 27;47:191-206. doi: 10.1016/j.jot.2024.06.009. eCollection 2024 Jul. J Orthop Translat. 2024. PMID: 39040489 Free PMC article. Review.
-
Electromagnetic Cellularized Patch with Wirelessly Electrical Stimulation for Promoting Neuronal Differentiation and Spinal Cord Injury Repair.Adv Sci (Weinh). 2024 Aug;11(30):e2307527. doi: 10.1002/advs.202307527. Epub 2024 Jun 13. Adv Sci (Weinh). 2024. PMID: 38868910 Free PMC article.
-
Metformin carbon dots enhance neurogenesis and neuroprotection in Alzheimer's disease: A potential nanomedicine approach.Mater Today Bio. 2024 Nov 16;29:101347. doi: 10.1016/j.mtbio.2024.101347. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39850274 Free PMC article.
-
Multifunctional magneto-electric and exosome-loaded hydrogel enhances neuronal differentiation and immunoregulation through remote non-invasive electrical stimulation for neurological recovery after spinal cord injury.Bioact Mater. 2025 Feb 28;48:510-528. doi: 10.1016/j.bioactmat.2025.02.034. eCollection 2025 Jun. Bioact Mater. 2025. PMID: 40104021 Free PMC article.
References
-
- a) Madl C. M., Heilshorn S. C., Blau H. M., Nature 2018, 557, 335; - PMC - PubMed
- b) Yang X.‐T., Bi Y.‐Y., Chen E.‐T., Feng D.‐F., J. Neurosci. Res. 2014, 92, 148; - PubMed
- c) Baumann H. J., Betonio P., Abeywickrama C. S., Shriver L. P., Leipzig N. D., Bioconjug. Chem. 2020, 31, 2125. - PMC - PubMed
-
- a) Wu P., Tarasenko Y. I., Gu Y., Huang L. Y., Coggeshall R. E., Yu Y., Nat. Neurosci. 2002, 5, 1271; - PubMed
- b) Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O., Nat. Med. 2002, 8, 963; - PubMed
- c) Guo R., Liao M., Ma X., Hu Y., Qian X., Xiao M., Gao X., Chai R., Tang M., J. Mater. Chem. B 2021, 9, 7793. - PubMed
-
- a) Mehlenbacher R. D., Kolbl R., Lay A., Dionne J. A., Nat. Rev. Mater. 2017, 3, 1;
- b) Lee J.‐U., Shin W., Lim Y., Kim J., Kim W. R., Kim H., Lee J. H., Cheon J., Nat. Mater. 2021, 20, 1029; - PubMed
- c) He L., Sun Z., Li J., Zhu R., Niu B., Tam K. L., Xiao Q., Li J., Wang W., Tsui C. Y., Hong Lee V. W., So K. F., Xu Y., Ramakrishna S., Zhou Q., Chiu K., Biomaterials 2021, 268, 120585; - PubMed
- d) Willand M. P., Nguyen M.‐A., Borschel G. H., Gordon T., Neurorehabil. Neural Repair 2016, 30, 490. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
