Chronotherapy based on modified-release hydrocortisone to restore the physiological cortisol diurnal rhythm
- PMID: 35618893
- PMCID: PMC9726814
- DOI: 10.1007/s13346-022-01183-w
Chronotherapy based on modified-release hydrocortisone to restore the physiological cortisol diurnal rhythm
Abstract
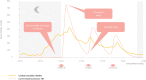
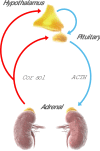
In this inspirational note, we describe the development of an endocrine chronotherapy to restore the physiological rhythm of the essential adrenal stress hormone, cortisol. The challenges included demonstrating the circadian rhythm of the drug target, creating a drug formulation that replicated that rhythm and then proving benefit in clinical trials. The physiological cortisol circadian rhythm is well defined with cortisol levels high on waking and low on going to sleep. We experimented with different formulation technologies including modified-release tablets and multi-particulates to replicate the cortisol rhythm where absent through disease. We describe the development of Efmody®, a modified-release formulation of hydrocortisone, which replicates the cortisol diurnal rhythm and improves the disease control of congenital adrenal hyperplasia, the commonest hereditary form of adrenal insufficiency. This program shows it is possible, through modified-release technology, to treat chronic endocrine diseases with physiological replacement to preserve health for life.
Keywords: Chronotherapy; Circadian; Cortisol; Efmody; Hormone; Hydrocortisone; Multi-particulate.
© 2022. The Author(s).
Conflict of interest statement
RJR is a director; MJW is an employee; and HH is a consultant to Diurnal Ltd.
Figures


References
-
- Jenkins-Jones S, Parviainen L, Porter J, Withe M, Whitaker MJ, Holden SE, Morgan CL, Currie CJ, Ross RJM. Poor compliance and increased mortality, depression and healthcare costs in patients with congenital adrenal hyperplasia. Eur J Endocrinol. 2018;178:309–320. doi: 10.1530/EJE-17-0895. - DOI - PubMed
-
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and treatment of primary adrenal insufficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:364–389. doi: 10.1210/jc.2015-1710. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

