COVID-19 diagnostic methods in developing countries
- PMID: 35619009
- PMCID: PMC9135468
- DOI: 10.1007/s11356-022-21041-z
COVID-19 diagnostic methods in developing countries
Retraction in
-
Retraction Note: COVID-19 diagnostic methods in developing countries.Environ Sci Pollut Res Int. 2025 May;32(22):13523-13524. doi: 10.1007/s11356-025-36527-9. Environ Sci Pollut Res Int. 2025. PMID: 40381079 Free PMC article. No abstract available.
Abstract
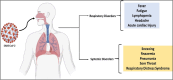
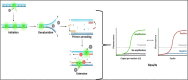
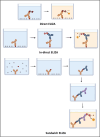
COVID-19 has become one of the few leading causes of death and has evolved into a pandemic that disrupts everyone's routine, and balanced way of life worldwide, and will continue to do so. To bring an end to this pandemic, scientists had put their all effort into discovering the vaccine for SARS-CoV-2 infection. For their dedication, now, we have a handful of COVID-19 vaccines. Worldwide, millions of people are at risk due to the current pandemic of coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2). Despite the lack of clinically authorized antiviral medications and vaccines for COVID-19, clinical trials of many recognized antiviral agents, their combination, and vaccine development in patients with confirmed COVID-19 are still ongoing. This discovery gave us a chance to get immune to this disease worldwide and end the pandemic. However, the unexpected capacity of mutation of the SARS-CoV-2 virus makes it difficult, like the recent SAS-CoV-2 Omicron variant. Therefore, there is a great necessity to spread the vaccination programs and prevent the spread of this dreadful epidemic by identifying and isolating afflicted patients. Furthermore, several COVID-19 tests are thought to be expensive, time-consuming, and require the use of adequately qualified persons to be carried out efficiently. In addition, we also conversed about how the various COVID-19 testing methods can be implemented for the first time in a developing country and their cost-effectiveness, accuracy, human resources requirements, and laboratory facilities.
Keywords: Antigen; Coronavirus; ELIZA; Immunity; RT-PCR; SARS-CoV-2; SHERLOCK; Viral invasion.
© 2022. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
Not Applicable.
Figures



References
-
- Amanat F, Stadlbauer D, Strohmeier S, et al (2020) A serological assay to detect SARS-CoV-2 seroconversion in humans. medRxiv: the preprint server for health sciences. 10.1101/2020.03.17.20037713
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

