Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study)
- PMID: 35619188
- PMCID: PMC9135390
- DOI: 10.1186/s13058-022-01530-2
Effect of physical exercise on cognitive function after chemotherapy in patients with breast cancer: a randomized controlled trial (PAM study)
Abstract
Background: Up to 60% of breast cancer patients treated with chemotherapy is confronted with cognitive problems, which can have a significant impact on daily activities and quality of life (QoL). We investigated whether exercise training improves cognition in chemotherapy-exposed breast cancer patients 2-4 years after diagnosis.
Methods: Chemotherapy-exposed breast cancer patients, with both self-reported cognitive problems and lower than expected performance on neuropsychological tests, were randomized to an exercise or control group. The 6-month exercise intervention consisted of supervised aerobic and strength training (2 h/week), and Nordic/power walking (2 h/week). Our primary outcome was memory functioning (Hopkins Verbal Learning Test-Revised; HVLT-R). Secondary outcomes included online neuropsychological tests (Amsterdam Cognition Scan; ACS), self-reported cognition (MD Anderson Symptom Inventory for multiple myeloma; MDASI-MM), physical fitness (relative maximum oxygen uptake; VO2peak), fatigue (Multidimensional Fatigue Inventory), QoL (European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ C-30), depression (Patient Health Questionnaire-9, Hospital Anxiety and Depression Scale; HADS), and anxiety (HADS). HVLT-R total recall was analyzed with a Fisher exact test for clinically relevant improvement (≥ 5 words). Other outcomes were analyzed using multiple regression analyses adjusted for baseline and stratification factors.
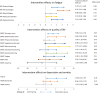
Results: We randomized 181 patients to the exercise (n = 91) or control group (n = 90). Two-third of the patients attended ≥ 80% of the exercise sessions, and physical fitness significantly improved compared to control patients (B VO2peak 1.4 ml/min/kg, 95%CI:0.6;2.2). No difference in favor of the intervention group was seen on the primary outcome. Significant beneficial intervention effects were found for self-reported cognitive functioning [MDASI-MM severity (B-0.7, 95% CI - 1.2; - 0.1)], fatigue, QoL, and depression. A hypothesis-driven analysis in highly fatigued patients showed positive exercise effects on tested cognitive functioning [ACS Reaction Time (B-26.8, 95% CI - 52.9; - 0.6) and ACS Wordlist Learning (B4.4, 95% CI 0.5; 8.3)].
Conclusions: A 6-month exercise intervention improved self-reported cognitive functioning, physical fitness, fatigue, QoL, and depression in chemotherapy-exposed breast cancer patients with cognitive problems. Tested cognitive functioning was not affected. However, subgroup analysis indicated a positive effect of exercise on tested cognitive functioning in highly fatigued patients. Trial Registration Netherlands Trial Registry: Trial NL5924 (NTR6104). Registered 24 October 2016, https://www.trialregister.nl/trial/5924 .
Keywords: Aerobic exercise; Breast cancer; Cancer-related cognitive impairment; Cognition; Cognitive complaints; Exercise training; Physical exercise; Physical fitness; Strength exercise.
© 2022. The Author(s).
Conflict of interest statement
The authors have declared no conflicts of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

