Phase 2 results of lisocabtagene maraleucel in Japanese patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma
- PMID: 35619325
- PMCID: PMC9761090
- DOI: 10.1002/cam4.4820
Phase 2 results of lisocabtagene maraleucel in Japanese patients with relapsed/refractory aggressive B-cell non-Hodgkin lymphoma
Abstract
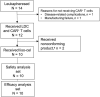
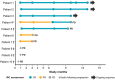
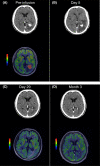
The autologous anti-CD19 chimeric antigen receptor (CAR) T-cell product, lisocabtagene maraleucel (liso-cel), is administered at equal target doses of CD8+ and CD4+ CAR+ T cells. This analysis assessed safety and efficacy of liso-cel in Japanese patients with relapsed or refractory (R/R) aggressive large B-cell lymphoma (LBCL) in Cohort 3 of TRANSCEND WORLD (NCT03484702). Liso-cel (100 × 106 total CAR+ T cells) was administered 2-7 days after lymphodepletion. The primary efficacy endpoint was objective response rate (ORR; Lugano 2014 criteria) assessed by an independent review committee. Fourteen patients were enrolled; 10 received liso-cel infusion (median time to liso-cel availability, 23 days) and were evaluable at data cutoff (median follow-up, 12.5 months). Grade ≥ 3 treatment-emergent adverse events were neutropenia (90%), leukopenia (80%), anemia (70%), and thrombocytopenia (70%). All-grade cytokine release syndrome (CRS) was observed in 50% of patients, though no grade ≥3 CRS events were reported. Grade 1 neurological events occurred in 1 patient but were resolved without any intervention. Prolonged cytopenia (grade ≥ 3 at day 29) was reported for 60% of patients. The ORR was 70%, and complete response rate was 50%. The median duration of response was 9.1 months (95% confidence interval [CI], 2.1-not reached), and overall survival was 14.7 months (95% CI, 1.7-not reached). One patient diagnosed with central nervous system involvement after screening but before liso-cel infusion, responded to liso-cel. Liso-cel demonstrated meaningful efficacy and a manageable safety profile in Japanese patients with R/R LBCL.
Keywords: Japanese patients; large B-cell lymphoma; lisocabtagene maraleucel; non-Hodgkin lymphoma; relapsed/refractory.
© 2022 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Shinichi Makita has received honoraria from Celgene, a Bristol‐Myers Squibb Company, Chugai, Daiichi Sankyo, Eisai, Novartis, and Takeda. Go Yamamoto, Dai Maruyama, Yuki Asano‐Mori, and Daisuke Kaji have no conflicts to report. Revathi Ananthakrishnan, Ken Ogasawara, and Lara Stepan are employees of Bristol Myers Squibb and may own stock in Bristol Myers Squibb. Jens Hasskarl was an employee of Celgene, a Bristol‐Myers Squibb Company, at the time of this analysis and may own stock in Bristol Myers Squibb. Nils Rettby and Claudia Schusterbauer are employees of Celgene, a Bristol‐Myers Squibb Company, and may own stock in Bristol Myers Squibb. Koji Izutsu has received research funding from Celgene, a Bristol‐Myers Squibb Company.
Figures


References
-
- Aoki R, Karube K, Sugita Y, et al. Distribution of malignant lymphoma in Japan: analysis of 2260 cases, 2001‐2006. Pathol Int. 2008;58:174‐182. - PubMed
-
- Miyoshi H, Ohshima K. Epidemiology of malignant lymphoma and recent progress in research on adult T‐cell leukemia/lymphoma in Japan. Int J Hematol. 2018;107:420‐427. - PubMed
-
- Suzuki T, Maruyama D, Maeshima AM, et al. Impact of the double expression of MYC and BCL2 on outcomes of primary refractory diffuse large B‐cell lymphoma following R‐CHOP chemoimmunotherapy. Blood. 2017;130:2846.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

